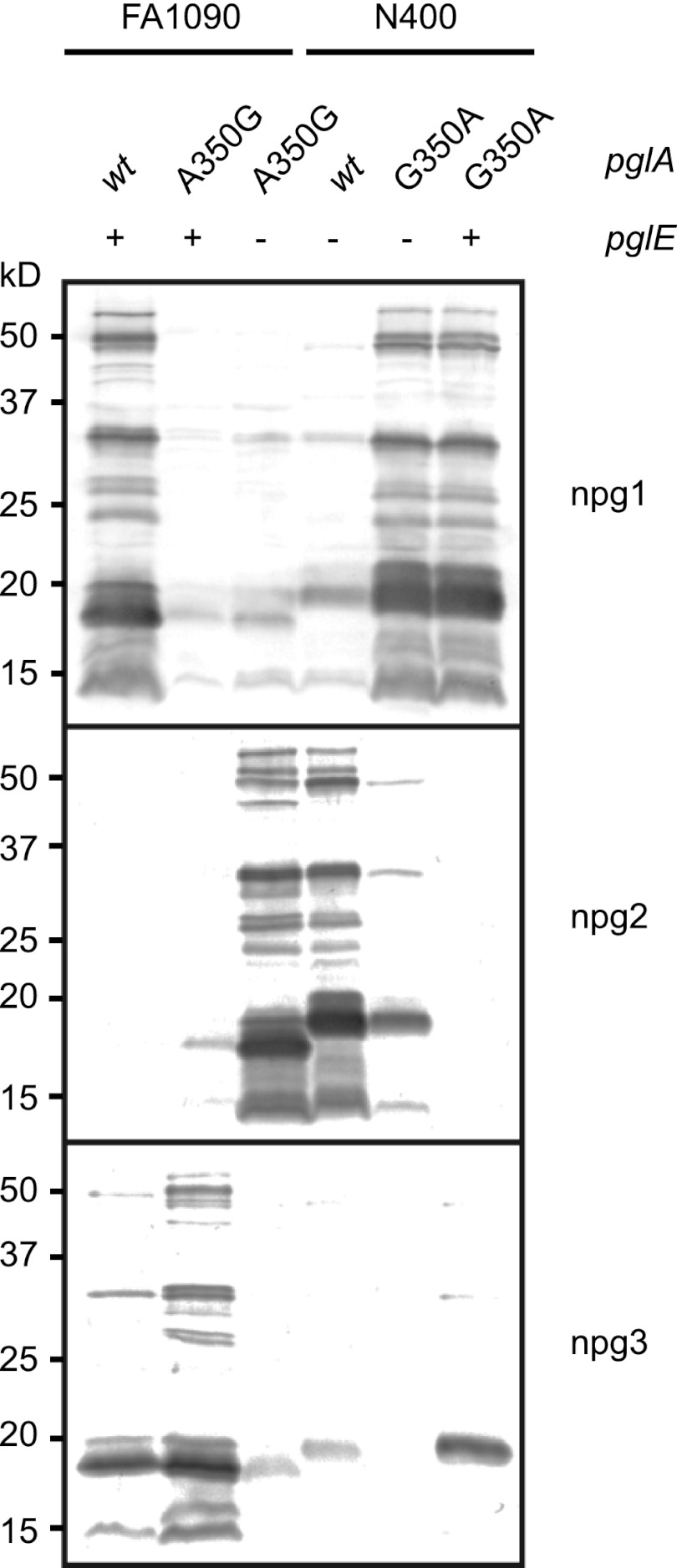
Fig 5.
Validation of the roles of amino acid 350 substitutions in common pglE backgrounds. Glycosylation patterns were monitored by immunoblotting with the glycan-specific antibodies npg1, npg2, and npg3. By introducing the A350G PglA mutation in the FA1090 pglEoff background, the obtained glycosylation was identical to that of N400 wild type. Similarly, by introducing the G350A PglA mutation in the N400 pglEon background, the achieved glycosylation was identical to that of FA1090 wild type. −, pglE from strain N400, which is pglEoff; +, pglE from strain FA1090, which is pglEon. Strains used were KS300, KS490, KS494, KS100, KS492, and KS493.