Fig 3.
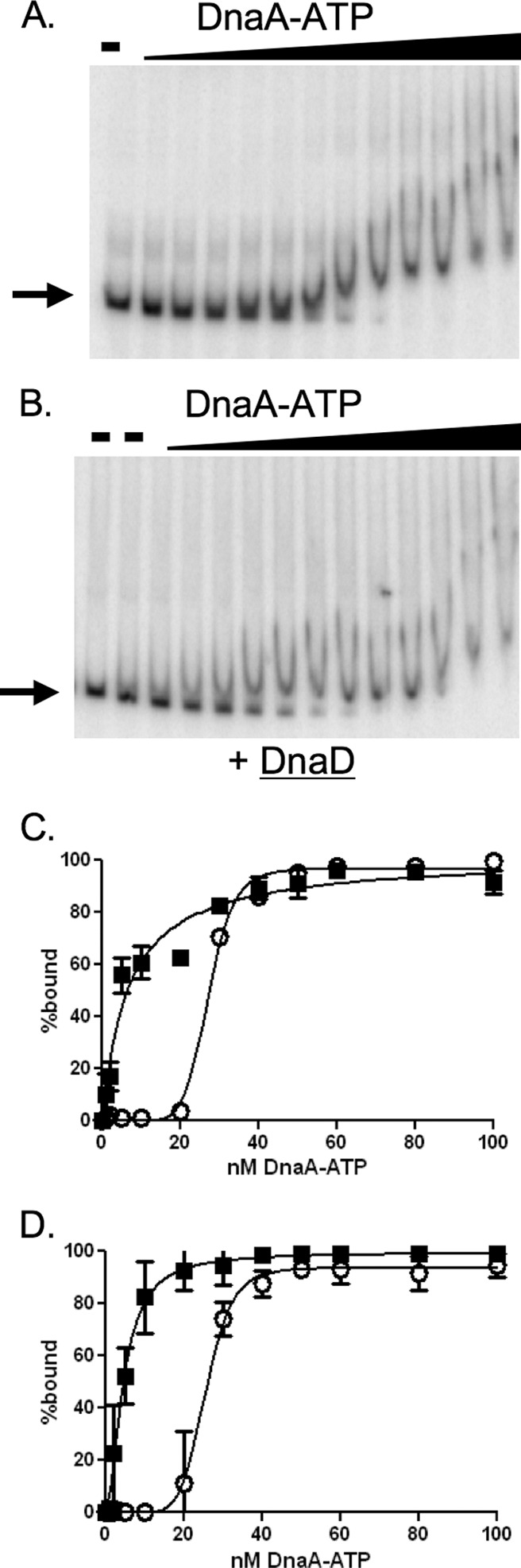
DnaD inhibits cooperative binding of DnaA to DNA. Representative gels and binding curves measuring binding of DnaA-ATP to DNA (50 pM) with and without purified DnaD-His6 (300 nM) are shown. DnaA concentrations used were 0, 1, 2, 5, 10, 20, 30, 40, 50, 60, 80, 100, and 200 nM. (A and B) Representative gels with increasing concentrations of DnaA-ATP incubated with template DNA from the oriC region in the absence (A) or presence (B) of DnaD-His6. Probe with no added protein is shown in the first lane of both panels. Probe with DnaD-His6 and no DnaA is shown in the second lane of panel B. (C and D) Data from three independent gel shift assays using template DNA from the oriC region (C) or the yydA region (D) are plotted as percent DNA bound versus the concentration of DnaA-ATP, in the absence (open circles) and presence (filled squares) of DnaD-His6. In experiments with the DNA fragment from the oriC region (C), the calculated Hill coefficient for DnaA-ATP was 8.6 in the absence of DnaD-His6 and 1 in the presence of DnaD-His6. The apparent Kd for DnaA-ATP was 27 nM in the absence and 6.6 nM in the presence of DnaD-His6. In experiments with the DNA fragment from the yydA region (D), the calculated Hill coefficient for DnaA-ATP was 6 in the absence of DnaD-His6 and 1 in the presence of DnaD-His6. The apparent Kd for DnaA-ATP was 25 nM in the absence and 5 nM in the presence of DnaD-His6.
