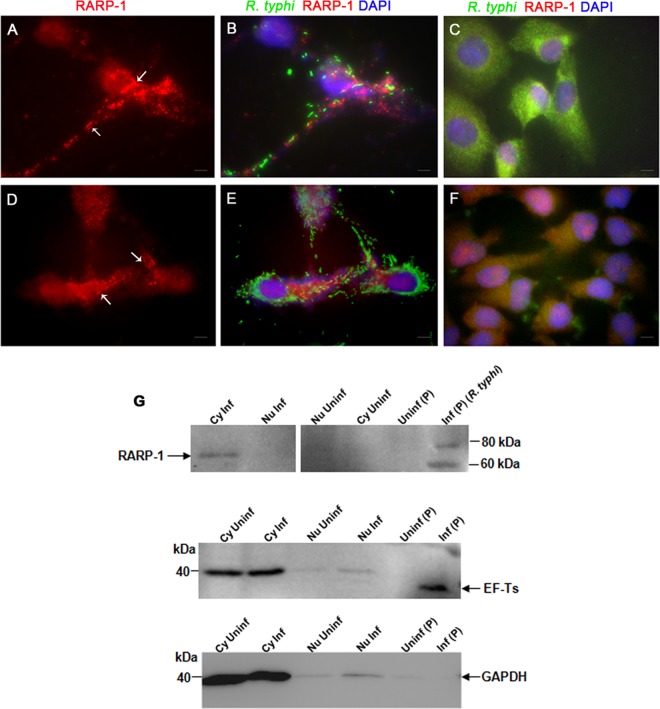
Fig 4.
Translocation of RARP-1 into host cells. Vero76 cells (A and B) and HeLa cells (D and E) were fixed with 4% paraformaldehyde at 24 h postinfection and immunolabeled using anti-RT0218 antibodies and anti-R. typhi rat serum as primary antibodies. The anti-rat-Alexa Fluor-488 (green) and anti-rabbit-Alexa Fluor-594 (red) were used as secondary antibodies. The cell nuclei are stained with DAPI (blue). The immunolabeled uninfected Vero76 and HeLa cells are shown in panels C and F, respectively. The white arrows show punctate structures indicating RARP-1 in the host cell cytoplasm. Scale bar, 5 μm. (G) Immunoblotting of the subcellular protein fractions (cytoplasmic [Cy], nuclear [Nu], and pellet [P]) of infected (MOI of ∼100) or uninfected Vero76 cells using anti-RT0218 antibodies. Controls included a blot probed with anti-EF-Ts (rickettsial housekeeping cytoplasmic protein) antibodies or monoclonal anti-GAPDH antibodies. The arrow indicates the secreted RARP-1 band with a molecular mass slightly smaller than the band detected in the pellet fraction containing rickettsial cell lysate, due to either nonspecific degradation or proteolytic processing of the protein. The faint band of EF-Ts (∼34 kDa) in the nuclear protein fraction indicates a minor carryover from the pellet fraction containing rickettsiae. The presence of GAPDH (∼36 kDa) in the nuclear fractions may be due to the presence of residual cytoplasmic proteins.