Figure 2. Purification of recombinant TDPX1 from E. coli.
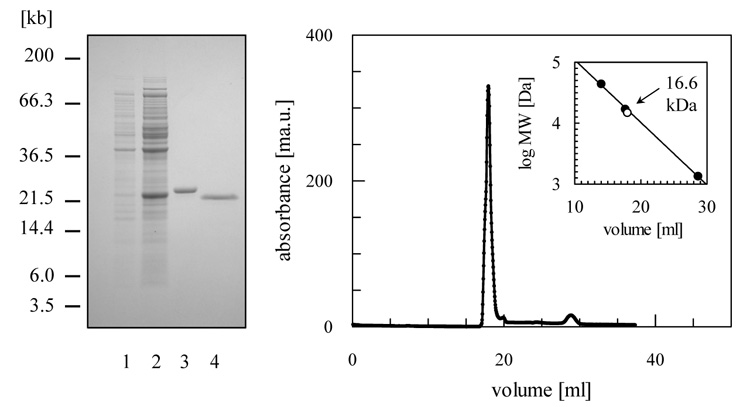
(A) SDS-PAGE analysis: lane 1, un-induced fraction of BL21 Star (DE3) pLysS (pET-15b – LmjF26.0820); lane 2, 4 h after induction with isopropyl-β-D-thiogalactopyranoside; lane 3, 2 μg of (His)6--tagged protein after chromatography on a nickel-chelating Sepharose column; lane 4, 2 μg of LmTDPX1 after removal of (His)6-tag with thrombin. (B) Gel filtration profile of LmTDPX1. The inset shows a plot of elution volume versus log molecular mass of a standard protein mixture (closed circles; ovalbumin, 44 kDa; myoglobin, 17 kDa; Vitamin B-12, 1.35 kDa). The open circle represents the elution volume of LmTDPX1.
