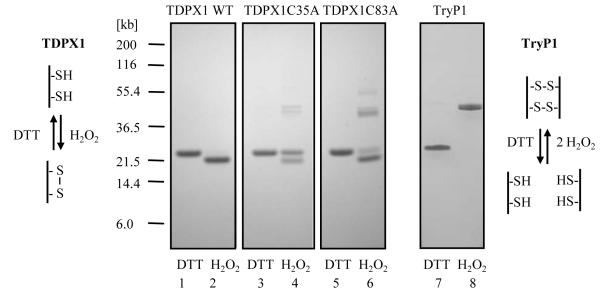
Figure 7. SDS-PAGE analysis of reduced and oxidized TDPX1, TDPX1 mutants and TryP1.
Proteins were first reduced with DTT or oxidized with H2O2 and then residual sulphydryl groups were alkylated with iodoacetamide as described in the experimental procedures. Aliquots (2 μg per lane) were separated by SDS-PAGE and stained with Coomassie: lanes 1 and 2, TDPX1 wild type; lanes 3 and 4, TDPX1 Cys35Ala; lanes 5and 6, TDPX1 Cys83Ala; lanes 7 and 8 TryP1 wild type. Odd numbered lanes are reduced with DTT and even numbered lanes oxidized with H2O2. The schematics show the predicted disulphide bond arrangement for TDPX1 and TryP1.