Table 2.
Interceptive decarboxylative allylation of allyl nitroalkanoates with Meldrum’s acid derived Michael acceptors.
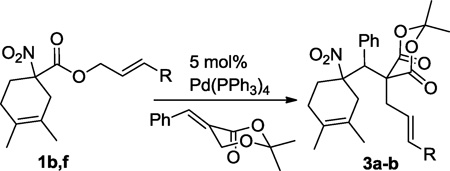 | |||||
|---|---|---|---|---|---|
| Entry | R | Solvent | Temp (°C) |
Time (h) |
% Yield (dr)a |
| 1 | H | DCM | rt | 12 | trace |
| 2 | H | DCM | 40 | 12 | trace |
| 3 | H | DCE | 80 | 12 | trace |
| 4 | H | THF | rt | 12 | 74 (7:3) |
| 5 | H | Tol | rt | 12 | 70 (7:3) |
| 6 | n-Pr | THF | 60 | 1 | 55 (7:3) |
The relative configuration of the major diastereomer is not known.
