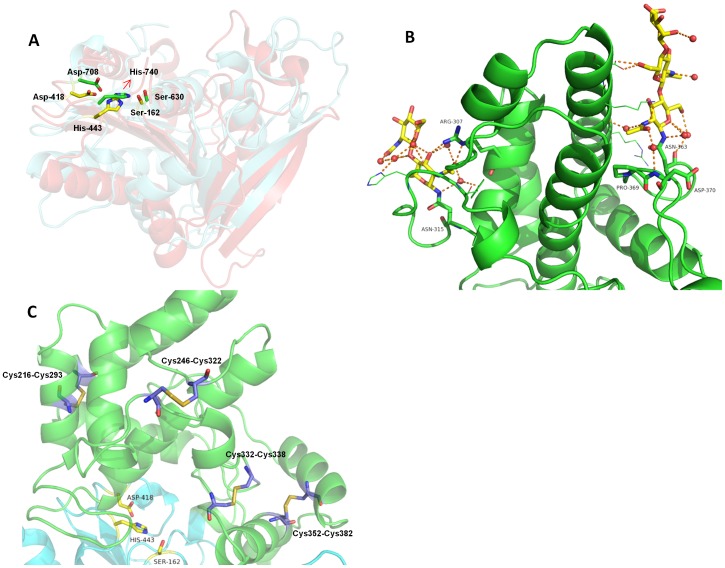
Figure 3. The catalytic domain and SKS domain.
SKS domain shown in green cartoon and the catalytic domain shown in aquamarine cartoon. The catalytic triad is represented as yellow sticks. (A) Conservation of the catalytic triad evidenced by the structural superposition. The catalytic fold of DPP4 is shown as red cartoon. DPP7 catalytic residues (Ser-162, Asp-418 and His-443) are colored in yellow, while DPP4 catalytic residues (Asp-708, His-740 and Ser-630) in green. The corresponding amino acid numbers are shown in black. (B) The SKS domain is shown in green cartoon, while the glycans linked to Asn363 and Asn315 are shown as sticks. Both glycans seem to have a scaffold function by holding the loop Glu354-Asp375 to the helix α8 and the loop Asp324-Tyr314 to the helix α12, respectively. (C) Four disulfide bonds present in the SKS domain. Cys216-Cys293 and Cys246-Cys322 seem to have a structure stabilization role. Cys332-Cys338 is very likely involved in the activity regulation of the enzyme, while Cys352-Cys382 could play either regulatory and structural role. The figure was prepared using the program PyMOL (http://www.pymol.org/).