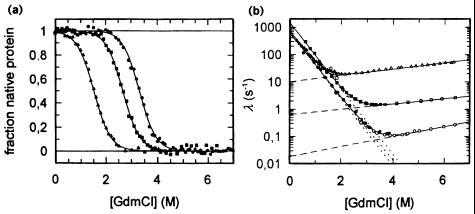
Figure 2.
Unfolding/folding mechanism of the Csp from Bacillus subtilis, Bacillus caldolyticus, and Thermotoga maritima with Topt= 52, 72, and 90°C, respectively. (a) Guanidinium chloride (GdmCl)-induced equilibrium unfolding transitions at 25°C, monitored by intrinsic fluorescence. Least-squares fit according to two-state model U ↔ N yields ΔGstab = 11.3, 20.1, and 26.2 kJ/mol for B. subtilis Csp, B. caldolyticus Csp, and T. maritima Csp, respectively. (b) Unfolding kinetics (open symbols) and refolding kinetics (closed symbols) of B. subtilis Csp (▵, ▴), B. caldolyticus Csp (□, ■), and T. maritima Csp (○, ●), respectively. The apparent rate constant λ is plotted against the denaturant concentration. Fits according to the two-state model (5, 11). [Reproduced with permission from ref. 11 (Copyright 1998, Nat. Struct. Biol.).]