Abstract
Purpose
To detect incidences and the types of chromosomal abnormalities in Chinese men with infertility and determine chromosomal factors association with various phenotypes.
Methods
Semen analysis and karyotype analysis by G-banding were carried out in 4,659 idiopathic infertile males; additionally, multiplex PCR using nine specific sequence-tagged sites (STSs) was used to detect azoospermia factor (AZF) microdeletions in 412 patients with Y chromosomal abnormalities.
Results
Male infertility was divided into pregestational infertility, characterized by failure to produce a fertilized ovum, and gestational infertility, characterized by embryo loss after fertilization. The former can result from azoospermia, oligozoospermia or oligoasthenozoospermia syndrome, while the latter is associated with developmental early pregnancy loss, habitual miscarriage and stillbirth. Among 4,659 male patients, 412 (8.84 %) showed abnormal chromosomal karyotypes, including 314 (6.74 %) with sex chromosomal abnormalities and 98 (2.10 %) with autosomal abnormalities. The prevalences of numerical and structural abnormalities among patients with chromosomal abnormalities were 259/412 (62.86 %) and 153/412 (37.14 %), respectively. Furthermore, structural sex chromosomal abnormalities were represented by various phenotypic profiles (46,XX, 47,XYY and 45,X/46,XY), and a prevalence of AZF microdeletions of 19/79 (24.05 %). AZF microdeletions were highly associated with Y chromosomal abnormalities (P = 0.018).
Conclusion
Various chromosomal abnormalities that result in male infertility could affect spermatogenesis or embryonic development at different levels. Sex chromosomal and autosomal abnormalities were highly associated with pregestational and gestational infertility, respectively. AZF microdeletions may play an important role in lowering the stability of the Y chromosome.
Keywords: Assisted reproductive technology, Azoospermia factor microdeletion, Chromosomal abnormality, Male factor infertility, Phenotype, Pregestational, Gestational infertility
Introduction
According to the World Health Organization, 10–15 % of couples of reproductive age are affected by infertility, and 40–50 % of these cases are associated with acquired and idiopathic male infertility factors. Moreover, chromosomal abnormalities are the predominant idiopathic factor [11]. Progress during the past few years has shown that infertile men have an 8–10-fold higher prevalence of chromosomal abnormalities than fertile men [17]. Also, with more than 2,300 genes associated with male germ-cell-specific transcripts and fertilization, spermatogenesis represents a very complex cell differentiation process [19]. This is particularly notable in the azoospermia factor (AZF) region of the Y chromosome. Recent studies have reported that AZF microdeletions are associated with chromosomal abnormalities, and have suggested that the detection of microdeletions should be performed routinely in all infertile males with Y chromosomal abnormalities [12, 20]. Similarly, a large number of genes are involved in the regulation of pre-implantation embryo survival and post-implantation embryonic development [9].
Assisted reproductive technology (ART), including in vitro fertilization with or without intracytoplasmic sperm injection and superovulation with intrauterine insemination, have been used to help produce healthy offspring, but the diversity of chromosomal abnormalities and the numerous impaired reproductive outcomes associated with these methods make cytogenetic analysis an indispensable test to guide the ART practitioner in their choice of techniques.
In this study, we present the cytogenetic results from 4,659 men from northeast China with proven infertility. Associations between the phenotypes of the individuals and chromosomal abnormalities were investigated. The results could help with the development of a more efficient screening program to benefit prospective patients, and provide information to assist with genetic counseling and the selection of more appropriate therapies.
Materials and methods
Patients
A total of 4,659 infertile men aged of 21–42 years (average 28 years) were recruited from the First Bethune Hospital of Jilin University (Changchun, China) from May 2004 to June 2011. Detailed information on patient and family medical histories, environmental details, relevant daily habits, genetic relationship with wife, and previous reproductive problems was collected for each patient to confirm that infertility was caused by chromosomal abnormalities. Anatomical factors and inflammation of the reproductive system were also ruled out by subjecting all couples to a physical examination. Patients’ wives were asked to provide gynecologic histories, including the results of karyotype analysis, and gynecological examination showed that all women were normal. The duration of infertility ranged from 1 to 8 years.
Karyotype analysis
Karyotyping of infertile men with unexplained spermatogenic disorders or whose partners experienced unexplained embryonic developmental failures (n = 4,659) was performed by G-banding of peripheral blood lymphocytes, according to standard techniques [10]. Briefly, peripheral blood lymphocytes were cultured for 72 h in RPMI medium 1640 (Gibco, Invitrogen Corporation, USA), and phytohemagglutinin (Shanghai Yihua Medical Technology Co., Ltd., China) containing fetal bovine serum (Beijing Dingguo Biotechnology, China), and then treated with colcemid (Sigma, UK). G-banding of metaphase chromosomes was performed by Giemsa staining. For each patient, the numbers of chromosomes in 20 metaphase mitotic figures were counted and the karyotypes of 10 cells in mitosis metaphase were analyzed. The procedure was repeated in all cases with abnormal karyotypes. Chromosomal abnormalities were described according to the International System of Cytogenetics Nomination [8].
Detection of AZF microdeletions
The detection of AZF microdeletions was performed by multiplex polymerase chain reaction (PCR). With reference to the microdeletion molecular diagnostic criteria published by the European Academy of Andrology and European Molecular Genetics Quality Network, a series of 10 specific sequence-tagged sites were mapped in the AZF region, including SY84 and SY86 for AZFa, SY27, SY134 and SY143 for AZFb, SY 157, SY254 and SY255 for AZFc, SY 152 for AZFd. Sex-determining region Y (SRY) and the human zinc-finger protein-encoding genes (ZFX/ZFY) located on the X and Y chromosomes were selected as internal control primers [4].
Two microliters of peripheral blood was extracted from each patient and anticoagulated with EDTA for PCR. Genomic DNA was separated from peripheral blood lymphocytes using a blood DNA extraction mini kit (Beijing Tiangen Biotech Co., Ltd., China).
Multiplex PCR amplification was performed in a 10-μl reaction system using Amplitron II PCR apparatus (Barnstead/Thermolyne, USA), containing 200 ng of genomic DNA, 1.5 mmol/l Mg2+, 800 μmol/l deoxyribonucleotide triphosphates, 10 pmol/l of each primer and 2 U Taq polymerase. Thermocycling (Veriti Thermal Cycler 96-well, Alpha-SE, Applied Biosystems, USA) consisted of predegeneration for 6 min at 94°C, followed by 35 cycles of 94°C for 40 s, 55°C for 45 s and 72°C for 60 s, with a final extension at 72°C for 6 min. After completion of the PCR reaction, the PCR product was removed and mixed with 2 μl of 6× buffer solution, then dropped onto a 1.5 % agarose gel (with 0.5 μg/ml Ethidium bromide). Electrolysis was performed at 80 V for 35 min, and the results were observed using an ultraviolet transmissometer. Cases detected as having AZF microdeletions were confirmed by repeating the above test. Thirty fertile men and 30 women were use as positive and negative controls, respectively, and water was used as a blank control.
Statistical analysis
Statistical analysis was carried out using SPSS 13.0 (Chicago, IL, USA). χ2 tests were used to compare phenotypes associated with sex chromosomal and autosomal abnormalities. Pearson's correlation analysis using a crosstabs table format was performed to detect the relationship between AZF microdeletions and Y chromosomal abnormalities. Statistical significance was assessed at P < 0.05, and all reported P values were two-sided.
Results
Clinical findings
Conventional semen analysis, patient questioning and ultrasonic testing of 4,659 patients with male factor infertility identified 2,139 (45.91 %) with azoospermia (AS) (no sperm in the ejaculate even after centrifugation), 814 (17.47 %) with oligozoospermia (OS) (sperm concentration <20 × 106/ml), and 182 (3.91 %) with oligoasthenozoospermia (OAT) syndrome (sperm count <20 × 106/ml, motility <40 % and normal forms <40 %), 99 (2.12 %) with developmental early pregnancy loss, 473 (10.15 %) with habitual miscarriage, 283 (6.07 %) with stillbirth, and 669 (14.36 %) with sexual dysfunction and other diseases. A total of 412 infertile men who visited the Center of Reproduction Medicine at the First Bethune Hospital of Jilin University (Changchun, China) were initially identified as having chromosomal abnormalities by cytogenetic analysis.
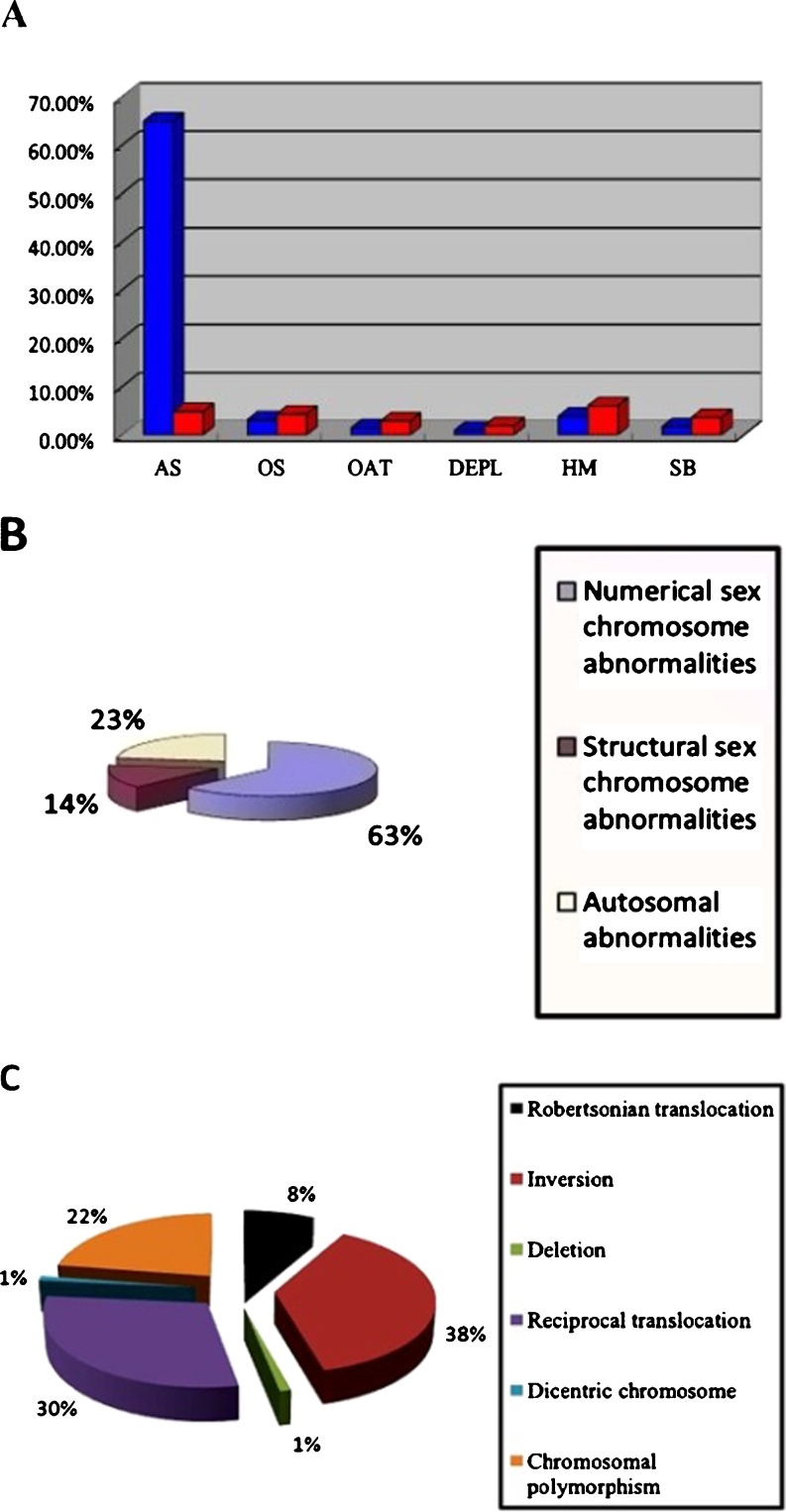
After exclusion of patients with obstruction of seminal ducts, retrograde ejaculation, genital tract inflammation, spermaduct, and genital tract and idiopathic epididymis inflammation, 412 (8.84 %) individuals with chromosomal abnormalities were selected for the study. This case group included 314 cases (76.21 %) with sex chromosomal abnormalities and 98 (23.79 %) with autosomal abnormalities. The proportions of patients with AS, OS, OAT syndrome, developmental early pregnancy loss, habitual miscarriage and stillbirth were 288 (69.90 %), 31 (7.52 %), 18 (4.37 %), 12 (2.91 %), 41 (9.95 %), and 22 (5.34 %), respectively (Fig. 1a).
Fig. 1.
Classification of infertile patients. a Clinical findings in 412 infertile males with chromosomal abnormalities. Blue and red pillars represent sex chromosomal and autosomal abnormalities, respectively. OAT oligoasthenozoospermia syndrome, DEPL developmental early pregnancy loss, HM habitual miscarriage, SB stillbirth. b Distribution of autosomal and sex chromosomal abnormalities in infertile males. c Distribution of types of structural autosomal abnormalities in 78 infertile males
Two by two comparisons of all clinical findings between sex chromosomal (n = 314) and autosomal abnormalities (n = 98) were performed using χ2 tests. The two-sided P value was <0.0001 in each case and was thus considered to be highly significant (Table 1).
Table 1.
Sex chromosomal abnormalities (n = 314) and corresponding clinical findings in men from northeast China with male factor infertility
| Clinical findings | |||||||
|---|---|---|---|---|---|---|---|
| Abnormal karyotype | Total | AS | OS | OAT syndrome | Developmental early pregnancy loss | Habitual miscarriage | Stillbirth |
| 47, XXY | 228 | 225 | 2 | 1 | |||
| 46,XY/47,XXY | 6 | 4 | 2 | ||||
| 46,XX/47,XXY | 1 | 1 | |||||
| 47, XYY | 5 | 1 | 1 | 2 | 1 | ||
| 46,XX | 6 | 6 | |||||
| 45,X/46,XY | 6 | 4 | 2 | ||||
| 46,XX/46,XY | 1 | 1 | |||||
| 45,X/46,XY,Yp+ | 1 | 1 | |||||
| 46,XY(Y ≤ 21) | 20 | 7 | 3 | 2 | 1 | 5 | 2 |
| 46,XY(Y ≥ 18) | 22 | 9 | 2 | 3 | 2 | 4 | 2 |
| 46,XY,Yp+ | 8 | 1 | 5 | 2 | |||
| 47,XY,+del(Yq12) | 1 | 1 | |||||
| 46,XX/47,XY,+del(Yq12) | 1 | 1 | |||||
| 46,XY,del(Yq) | 2 | 2 | |||||
| 46,X,+del(q11.21) | 1 | 1 | |||||
| 45,X,der(Y;22)(q10,q10) | 1 | 1 | |||||
| 45,X,-Y,-13,+t(Y;13) (p1;q13) | 1 | 1 | |||||
| 45,X,-Y,-15,+t(Y;15) (p1;q11) | 1 | 1 | |||||
| 46,XY,t(Y;4)(p11;p14) | 1 | 1 | |||||
| 46,XY,t(Y;14)(q11; p11) | 1 | 1 | |||||
| Total number | 314 | 268 | 13 | 6 | 4 | 16 | 7 |
| Proportion | 76.22 % | 65.05 % | 3.16 % | 1.46 % | 0.97 % | 3.88 % | 1.70 % |
The most prevalent phenotype among sex chromosomal abnormalities was AS, but the clinical distribution among autosomal abnormalities was more homogeneous, with habitual miscarriage being the most frequent at 6.07 % (25/412), and other clinical conditions varying from 1.94 % (developmental early pregnancy loss) to 4.85 % (AS) (Table 2).
Table 2.
Autosomal abnormalities and corresponding clinical findings in men from northeast China with male factor infertility
| Clinical findings | |||||||
|---|---|---|---|---|---|---|---|
| Abnormal karyotype | Total | AS | OS | OAT syndrome | Developmental early pregnancy loss | Habitual miscarriage | Stillbirth |
| 45,XY,der(13;14)(q10;p10) | 3 | 1 | 2 | ||||
| 45,XY,der(13;14)(q11;p11) | 1 | 1 | |||||
| 45,XY,der(14;21)(p10;p10) | 1 | 1 | |||||
| 45,XY,der(14;21)(q10;q10) | 2 | 2 | |||||
| 46,XY,inv(7)(p13q36) | 1 | 1 | |||||
| 47,XXY,inv(9)(p11q12) | 1 | 1 | |||||
| 46,XY,inv(9)(p11q13) | 4 | 2 | 1 | 1 | |||
| 46,XY,inv(9)(p11q12) | 30 | 8 | 4 | 6 | 2 | 8 | 2 |
| 46,XY,inv(1)(p36;q25)inv(9) (p11;q12) | 1 | 1 | |||||
| 46,XY,t(1;2)(q21;q37) | 2 | 1 | 1 | ||||
| 46,XY,t(1;4)(p36;q31) | 1 | 1 | |||||
| 46,XY,t(4;5)(q21;p15) | 1 | 1 | |||||
| 46,XY,t(1;3;6)(p32;q29;q14) | 1 | 1 | |||||
| 46,XY,t(7;15)(p15;q15) | 1 | 1 | |||||
| 46,XY,t(1;9)(p22;p24) | 1 | 1 | |||||
| 46,XY,t(4;9)(q35;p13) | 3 | 1 | 2 | ||||
| 46,XY,t(1;18)(p32;q23) | 2 | 2 | |||||
| 46,XY,-14,+t(14;21)(q10;q10) | 3 | 1 | 2 | ||||
| 46,XY,t(10;22)(q25;q13) | 1 | 1 | |||||
| 46,XY,-21,t(21;21)(q10;q10) | 1 | 1 | |||||
| 46,XY,t(4;13)(q12;q12) | 1 | 1 | |||||
| 46,XY,t(18;20)(p11;q11) | 1 | 1 | |||||
| 46,XY,t(1;13)(p22;q14) | 1 | 1 | |||||
| 45,XY,der(13;14)(q10;q10) | 1 | 1 | |||||
| 46,XY,t(4;17)(q21;p13) | 2 | 2 | |||||
| 46,XY,t(3;12)(q28;q15) | 1 | 1 | |||||
| 46,XY,t(6;10)(q23;p13) | 1 | 1 | |||||
| 46,XY,t(6;14)(q13;p10) | 1 | 1 | |||||
| 46,XY,t(8;11)(q24;q23) | 1 | 1 | |||||
| 46,XY,t(3;7)(q25;q22) | 1 | 1 | |||||
| 46,XY,t(2;4)(q31;q31) | 1 | 1 | |||||
| 46,XY,t(4;12)(p14;q13) | 1 | 1 | |||||
| 46,XY,del(4)(p15.3) | 1 | 1 | |||||
| 45,XY,-17,-22,+dic(17;22) (p13;q13) | 1 | 1 | |||||
| 46,XY(1q12+) | 4 | 3 | 1 | ||||
| 46,XY(1qh+) | 2 | 2 | |||||
| 46,XY(9q11+) | 1 | 1 | |||||
| 46,XY(9qh+) | 3 | 1 | 2 | ||||
| 46,XY(13s+) | 1 | 1 | |||||
| 46,XY(14s+) | 1 | 1 | |||||
| 46,XY(15s-) | 1 | 1 | |||||
| 46,XY(15pstk+) | 1 | 1 | |||||
| 46,XY(21s+) | 1 | 1 | |||||
| 46,XY(21ps-) | 1 | 1 | |||||
| 46,XY(21pstk+) | 1 | 1 | |||||
| 46,XY(22s+) | 3 | 1 | 2 | ||||
| 46,XY(22p+) | 2 | 2 | |||||
| Total number | 98 | 20 | 18 | 12 | 8 | 25 | 15 |
| Proportion in cohort (n = 412) | 23.78 % | 4.85 % | 4.37 % | 2.91 % | 1.94 % | 6.07 % | 3.64 % |
Numerical sex chromosomal abnormalities were the most common (Fig. 1b). However, all autosomal abnormalities were structural, including Robertsonian translocations, reciprocal translocations, deletions, pericentric inversions, dicentric chromosomes and chromosomal polymorphisms, with reciprocal translocations and inversions being the most common (Fig. 1c).
Chromosomal microdeletions in the AZF region
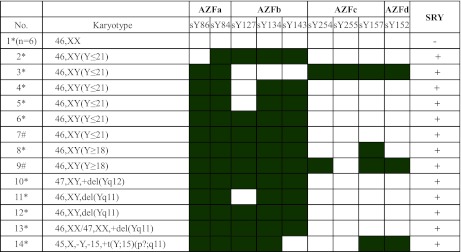
Nineteen (4.61 %) of 412 patients with abnormal karyotypes had microdeletions in the AZF region. This included 17 males with AS and two with OS. Most of these karyotypes occurred in patients with structural Y chromosomal abnormalities. Interestingly, the proportion of AZF microdeletions was 24.05 % (19/79) among males with structural Y chromosomal abnormalities showing phenotypes (46,XX, 47,XYY or 45,X/46,XY) associated with the stability of the Y chromosome in the process of formation (Tables 1 and 3). Six (30 %) of 20 patients with 46,XY(Y ≤ 21) karyotypes and two (9.09 %) of 22 patients with 46,XY(Y ≥ 18) karyotypes had AZF microdeletions. Among these patients with AZF microdeletions, the most frequent microdeletions in 19 of them, except for those with phenotype AZFa+b+c+d, were in the AZFc+d region, followed by AZFb+c+d, AZFa+c+, AZFb+c, AZFb and AZFc (Table 3). Moreover, only one patient (No. 3) showed no AZFc microdeletion (Table 3), and no AZF microdeletions were detected in the fertile controls. The statistical data presented in Table 4 show a high correlation (r = 0.37, P = 0.018) between AZF microdeletions and Y chromosomal abnormalities.
Table 3.
Microdeletion of AZFs in 14 infertile men with chromosomal abnormalities
*Patients with AS; # patients with OS. Filled and blank cells signify absence or presence of sequence-tagged site microdeletions in AZF region, respectively. + and – signify positive and negative results of SRY by PCR, respectively
Table 4.
Correlation between AZF microdeletions and Y chromosomal abnormalities
| AZF | ||||
|---|---|---|---|---|
| Microdeletion | No microdeletion | Total | ||
| Y chromosome | Abnormal | 19 | 393 | 412 |
| Normal | 107 | 4140 | 4247 | |
| Total | 126 | 4533 | 4659 | |
r = 0.37, P = 0.018
Discussion
The results of the current study revealed that 8.84 % of a large cohort of 4,659 infertile male patients had chromosomal abnormalities, which was significantly higher than the prevalence (0.37 %) in 10,202 phenotypically normal and fertile adult males [14]. The prevalence of sex chromosomal abnormalities in male patients was about 2- to 3-fold greater than that of autosomal abnormalities. In addition, in terms of the clinical findings, patients with AS were significantly more likely to have sex chromosomal abnormalities than autosomal abnormalities (65.29 % vs 4.85 %; P < 0.001). In contrast, patients with autosomal abnormalities were more likely to suffer from OS, OAT syndrome, developmental early pregnancy loss, habitual miscarriage and stillbirth (Fig. 1a). These results suggest that sex chromosomal abnormalities are more likely to be responsible for pregestational infertility, while autosomal abnormalities are more likely to result in gestational infertility.
47,XXY and its mosaics of different forms or ratios were the most frequently detected abnormal karyotypes. Klinefelter's syndrome accounted for 5.04 % of all infertile males, and 97.45 % of Klinefelter patients had AS, as did four out of six cases with karyotypes 46,XY/47,XXY. These findings are in accordance with previous reports where focal spermatogenesis failure and severe OS were found in cases with 46,XY(X)/47,XXY mosaic karyotypes [15]. The current study further supports the theory that a superabundance of X chromosomes could affect the male-determining function by inhibiting maturity, hence promoting degenerative changes in the testis convoluted tubule and thus affecting spermatogenesis, ultimately resulting in varying degrees of related androgen dysfunctions [13].
Y chromosome aneuploidies in patients in this study included 47,XYY (5 cases), 45,X/46,XY (6 cases), 45,X/46,XY, Yp+(1 case). The presence of an extra non-synapsed Y chromosome is thought to activate checkpoint mechanisms to prevent the progression of meiosis, leading to dyszoospermia [18]. Surprisingly, significant increases in XY, YY, chromosomes 13, 18 and 21 disomic and nullisomic sperm were observed in 47,XXY infertile men with increased XX disomy sperm [16]. Moreover, it is generally accepted that the recombined autosomal trisome affects pre-implantation embryo survival and post-implantation embryonic development, which can increase the possibility of early pregnancy loss, habitual miscarriage and stillbirth [5]. The loss of a Y chromosome could lead to AS or OS, depending on the ratio of the Y chromosome, in accordance with the gene (related to spermatogenesis) dose effect. In the current study, all six men with 46,XX sex reversal, as well as the one 46,XX/46,XY male, were diagnosed with AS. Furthermore, both SRY and AZF genes were absent in the 46,XX phenotype. In our cohort, we noted a 26-year-old patient carrier of 46,XX/47,XXY; however, this case is in conflict with the reported explanation that while the coincident phenomenon (46,XX/47,XXY) can be seen during embryonic development, the 47,XXY cell line disappears gradually with age.
It has been shown that some chromosomal polymorphisms can be associated with habitual miscarriage, developmental early pregnancy loss, stillbirth, poor maternal history and cacospermia [6]. In the present study, Y chromosomal polymorphisms included 46,XY(Y ≥ 18) (22 cases), 46,XY(Y ≤ 21) (20 cases) and 46,XY,Yp+(8 cases). Y(≥18) results from excessive Y-typical DY21 sequence duplication in the heterochromatin region, and is affiliated with errors during mitosis, gene regulation and cell differentiation, and leads to gestational problems. Y(≤21) is similar to the normal Y chromosome in morphology and bands, as well as in size. In the present study, 30 % of the males with 46,XY(Y ≤ 21) exhibited AZF microdeletions, highlighting the fact that the small Y chromosome could lack genes associated with spermatogenesis. Alternatively, the small Y could affect mitosis and cause sterility in a similar way to that seen with the larger Y chromosome.
In this study, the most frequent microdeletions among patients with abnormal karyotypes were located in the AZFc region. These findings are in agreement with those of previous reports, which associated AZF microdeletions with AS, OS and OAT syndrome [21], and also with chromosomal abnormalities. This suggests that genes in the AZF region are associated not only with spermatogenesis, but also with the stability and viability of the Y chromosome, such that microdeletions are an intrinsic element of the AZF gene polymorphism that can lead to translocations and deletions, as well as gain or loss of Y chromosomes.
Reciprocal and Robertsonian translocation carriers produce high-risk gametes that can combine with normal ova leading to gestational infertility. Furthermore, translocated chromosomes disturb and impede meiosis, resulting in varying extents of spermatogenic impairment [3, 17].
In the case of chromosomal inversions, the force and time restrictions imposed on the formation of a pairing loop can delay meiosis; moreover, reduced recombination within the pairing loop also leads to a breakdown of meiosis [1]. However, there is no loss of genetic material, and inversions therefore do not usually cause abnormalities in carriers, whereas abnormal gametes formed by meiosis may lead to gestational infertility in heterozygotes [2]. In the current study, 75 % (27/37) infertile men with chromosomal inversions had residual spermatogenesis. This supports the above statement, and furthermore, the appearance of different chromosomes and the location of breakpoints involved in inversions are not random [7]. Data from the present study showed that paracentric inversions occurred most frequently in chromosome 9, followed by chromosomes 1 and 7.
Autosomal polymorphisms mainly indicate mutations of the heterochromatin distributed in the centromere, telomere, or satellite and at secondary constrictions. The present study detected this type of chromosomal abnormality on chromosomes 1, 9, 12 and 22. Autosomal polymorphisms (45.44 %, 10/22) were associated with habitual abortion (45.44 %), AS (18 %) and stillbirth (18 %).
Conclusion
Chromosomal abnormalities were detected in 8.84 % of 4,659 infertile men in northeast China. Autosomal abnormalities were associated with gestational infertility, while sex chromosomal abnormalities were associated with pregestational infertility. Furthermore, there was a strong association between AZF microdeletions and chromosomal abnormalities. The results of this study will further our understanding of the etiology of infertility caused by paternal chromosomal abnormalities, and provide guidance to allow ART practitioners to make more appropriate therapeutic choices.
Acknowledgments
We would like to thank all the patients recruited for this study, especially the donors of DNA samples. We would also like to express our sincere gratitude to all the staff of the Andrology Laboratory for their excellent work. This work was kindly supported by funds from the National Population and Family Planning Commission of China (NO. 2011-GJKJS-07).
Footnotes
Capsule
AZF microdeletions are highly associated with Y chromosomal abnormalities. Sex chromosomal and autosomal abnormalities in men are prone to pregestational and gestational infertility respectively.
References
- 1.Anton E, Blanco J, Egozcue J, Vidal F. Risk assessment and segregation analysis in a pericentric inversion inv(6p23q25) carrier using FISH on decondensed sperm nuclei. Cytogenet Genome Res. 2002;97:149–154. doi: 10.1159/000066603. [DOI] [PubMed] [Google Scholar]
- 2.Bernicot I, Dechanet C, Mace A, Hedon B, Hamamah S, Pellestor F, et al. Predictive value of sperm-FISH analysis on the outcome of preimplantation genetic diagnosis (PGD) for a pericentric inversion inv5(p15.3q11.2) carrier. Hum Reprod. 2010;25(7):1818–1823. doi: 10.1093/humrep/deq101. [DOI] [PubMed] [Google Scholar]
- 3.Chantot-Bastaraud S, Ravel C, Siffroi JP. Underlying karyotype abnormalities in IVF/ICSI patients. Reprod. Biomed. 2008;16:514–522. doi: 10.1016/S1472-6483(10)60458-0. [DOI] [PubMed] [Google Scholar]
- 4.Dada R, Gupta NP, Kucheria K. Molecular screening for Yq microdeletion in men with idiopathic oligozoospermia and azoospermia. J Biosci. 2003;28(2):163–168. doi: 10.1007/BF02706215. [DOI] [PubMed] [Google Scholar]
- 5.Egozcue S, Blanco J, Vendrell JM, García F, Veiga A, Aran B, et al. Human male infertility: chromosome anomalies, meiotic disorders, abnormal spermatozoa and recurrent abortion. Hum Reprod Update. 2000;6(1):93–105. doi: 10.1093/humupd/6.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y, Zhou YW, Tao J, Wang SX, Zhao XM. Do polymorphic variants of chromosomes affect the outcome of in vitro fertilization and embryo transfer treatment? Hum Reprod. 2011;26(4):933–940. doi: 10.1093/humrep/deq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichioka K, Yoshimura K, Honda T, Takahashi A, Terai A. Paracentric inversion of chromosome 7(q22–31) associated with nonobstructive azoospermia. Fertil Steril. 2005;83:455–456. doi: 10.1016/j.fertnstert.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 8.International Standing Committee on Human Cytogenetic Nomenclature, Shaffer LG, Tommerup N. ISCN 2005: An International System for Human Cytogenetic Nomenclature (2005): Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature. Cytogenet Genome Res. 2005.
- 9.Levy R. Genetic regulation of preimplantation embryo survival. Int Rev Cyt. 2001;210:1–22. doi: 10.1016/S0074-7696(01)10002-1. [DOI] [PubMed] [Google Scholar]
- 10.Male Infertility Best Practice Policy Committee of the American Urological Association, Practice Committee of the American Society for Reproductive Medicine Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86:S202–S209. doi: 10.1016/j.fertnstert.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Mark SK, Renee A, Reijo P. Male infertility, genetic analysis of the DAZ genes on the human Y chromosome and genetic analysis of DNA repair. Mol Cell Endocrinol. 2001;184(1–2):41–49. doi: 10.1016/s0303-7207(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 12.Mitra A, Dada R, Kumar R, Gupta NP, Kucheria K, Gupta SK. Y chromosome microdeletion in azoospermic patients with Klinefelter's syndrome. Asian J Androl. 2006;8(1):81–88. doi: 10.1111/j.1745-7262.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 13.Koşar PA, Özçelik N, Koşar A. Cytogenetic abnormalities detected in patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet. 2011;27(1):17–21. doi: 10.1007/s10815-009-9366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravel C, Berthaut I, Bresson JL, Siffroi JP. Prevalence of chromosomal abnormalities in phenotypically normal and fertile adult males: large-scale survey of over 10,000 sperm donor karyotypes. Hum Reprod. 2006;21:1484–1489. doi: 10.1093/humrep/del024. [DOI] [PubMed] [Google Scholar]
- 15.Rives N, Joly G, Machy A, Siméon N, Leclerc P, Macé B. Assessment of sex chromosome aneuploidy in sperm nuclei from 47, XXY and 46, XY/47, XXY males: comparison with fertile and infertile males with normal karyotypes. Mol Hum Reprod. 2000;6:107–112. doi: 10.1093/molehr/6.2.107. [DOI] [PubMed] [Google Scholar]
- 16.Rives N, Simeon N, Milazzo JP, Barthélémy C, Macé B. Meiotic segregation of sex chromosomes in mosaic and non-mosaic XYY males: case reports and review of the literature. Int J Androl. 2003;26:242–249. doi: 10.1046/j.1365-2605.2003.00421.x. [DOI] [PubMed] [Google Scholar]
- 17.McLachlan RI, O’Bryan MK. State of the art for genetic testing of infertile men. J Clin Endocrinol Metab. 2010;95(3):1013–1024. doi: 10.1210/jc.2009-1925. [DOI] [PubMed] [Google Scholar]
- 18.Roeder GS, Bailis JM. The pachytene checkpoint. Trends Genet. 2000;16:395–403. doi: 10.1016/S0168-9525(00)02080-1. [DOI] [PubMed] [Google Scholar]
- 19.Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA. 2003;100(21):12201–12206. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umeno M, Shinka T, Sato Y, Xin-JunYang BY, Iwamoto T, Nakahori Y. A rapid and simple system of detecting deletions on the Y chromosome related with male infertility using multiplex PCR. J Med Invest. 2006;53(1–2):147. doi: 10.2152/jmi.53.147. [DOI] [PubMed] [Google Scholar]
- 21.Zamani AG, Kutlu R, Durakbasi-Dursan HG, Gorkemli H, Acar A. Y chromosome microdeletions in Turkish infertile men. Indian J Hum Genet. 2006;12:66–71. doi: 10.4103/0971-6866.27788. [DOI] [Google Scholar]