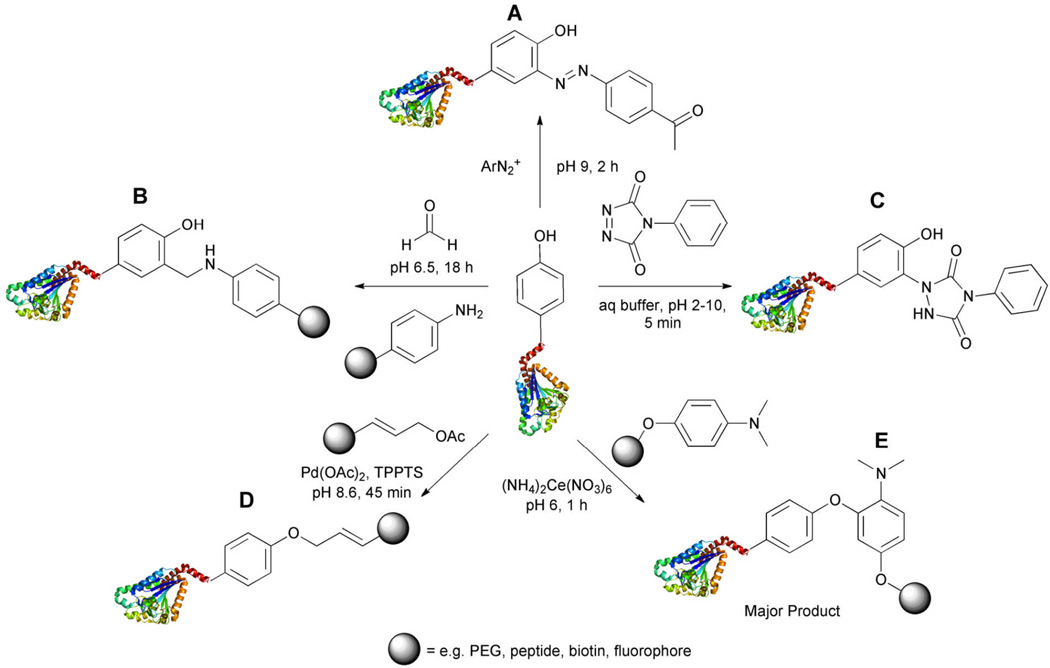
Figure 7.
Tyrosine residues have been targeted utilizing several different strategies, reaction with the electron-rich aromatic ring at the position ortho to the hydroxyl or direct targeting of the phenol hydroxyl group. A. Coupling to an electron deficient diazonium salt. B. A three component Mannich-type reaction yields an o-substituted tyrosine moiety. C. An ene-type reaction is performed between tyrosine and a cyclic diazocarboxamide yielding a highly stable product. D. Metal-mediated coupling facilitates reaction at the phenolic site. Palladium promotes addition of allylic substrates. E. Cerium promotes a one-electron oxidative coupling to tyrosine.