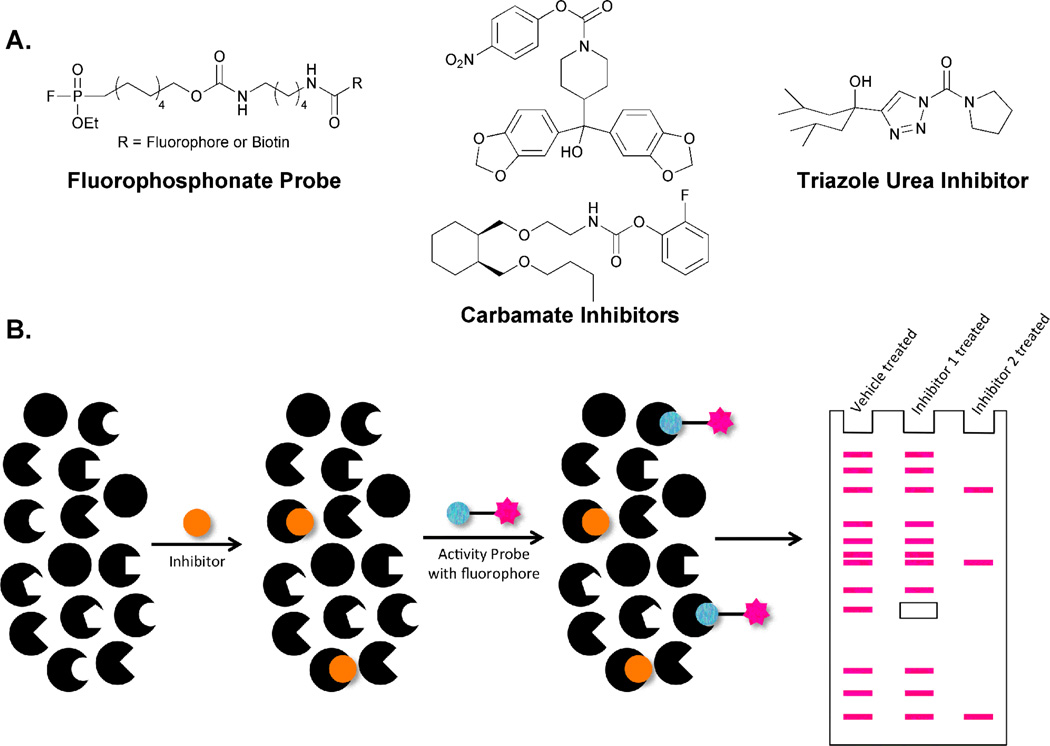
Figure 8.
Activity-based protein profiling can be used to target enzymes that utilize hydroxyl group-containing active site nucleophiles. A. Serine hydrolases can be targeted by several compound classes. Fluorophosphonate-based probes are promiscuous within the protein family. Compounds with attenuated reactivity such as carbamates and ureas enable selective examination of one or a small number of family members. B. The global nature of FP labeling can be utilized in the development of an assay to identify highly specific serine hydrolase inhibitors. Vehicle treated samples will display labeling of all FP-susceptible proteins. Addition of potential inhibitors will prevent susceptible enzymes from reacting with the FP probe, leading to a loss of a band or bands during gel-based analysis. Selective inhibitors will cause the loss of only one protein band (i.e., inhibitor 1) while promiscuous inhibitors will promote the loss of many (i.e., inhibitor 2).