Abstract
Closer relationships between caregivers and care recipients with dementia are associated with positive outcomes for care recipients, but it is unclear if closeness is a risk or protective factor for the health and psychological well-being of caregivers. We examined 234 care dyads from the population-based Cache County Dementia Progression Study. Caregivers included spouses (49%) and adult offspring (51%). Care recipients mostly had dementia of the Alzheimer’s type (62%). Linear mixed models tested associations between relationship closeness at baseline, or changes in closeness prior to versus after dementia onset, with baseline levels and changes over time in caregiver affect (Affect Balance Scale; ABS), depression (Beck Depression Inventory; BDI), and mental and physical health (components of the Short-Form Health Survey; SF-12). After controlling for demographic characteristics of the caregiver, number of caregiver health conditions, and characteristics of the care recipient (type of dementia, functional ability, and behavioral disturbances), we found that higher baseline closeness predicted higher baseline SF-12 mental health scores (better mental health), and lower depression. Higher baseline closeness also predicted greater worsening over time in ABS and SF-12 mental health. In addition, caregivers who reported a loss of closeness in their relationship with the care recipient from pre- to post-dementia displayed improved scores on ABS and SF-12 mental health, but worse SF-12 physical health over the course of the study. These results suggest that closeness and loss of closeness in the care dyad may be associated with both positive and adverse outcomes for caregivers, both cross-sectionally and over time.
The Stress Process model (Pearlin, Mullan, Semple, & Skaff, 1990) suggests that caregiving is fundamental to many dyadic relationships (marital, parent-child, etc.), but when one member of a dyad develops dementia, the relationship shifts from an equal exchange of assistance, to a greater burden on the caregiver. Closer relationships between caregivers and care recipients with dementia (the care dyad) serve a protective role for care recipients (Burgener & Twigg, 2002; Norton, et al., 2009; Perren, Schmid, Herrmann, & Wettstein, 2007; Whitlatch, Schur, Noelker, Ejaz, & Looman, 2001) but the literature is inconsistent on whether closeness serves a risk or protective role in the health and psychological well-being of caregivers. Addressing the emotional bond between the caregiver and care recipient is an integral part of counseling dementia caregivers (LoboPrabhu, Molinari, & Lomax, 2006), yet it remains unclear whether closer dyadic relationships or emotional detachment is physically and/or psychologically healthier for caregivers. The current study examines how closeness of the care dyad relationship relates to change over time in caregiver physical health and several indicators of psychological well-being. Such data could answer whether promoting closeness or promoting emotional detachment is recommended for care dyads.
As dementia-related care commences, dyadic relationships take on new roles and meanings, with potentially adverse outcomes. Comparative research demonstrated that Alzheimer’s disease (AD) caregivers reported fewer shared activities and less reciprocity in their relationships, compared to individuals in non-caregiving relationships (Gallagher-Thompson, Dal Canto, Jacob, & Thompson, 2001). The Stress Process model proposes that dementia caregivers are exposed to care-related stressors, increasing their risk of adverse psychological and physical health outcomes. One such stressor is “relational deprivation”, or the “deprivation of intimate exchange”, which includes losing the closeness of the relationship and a confidant as dementia progresses. As described by Pearlin and colleagues (1990), “The sheer dramatic and involuntary transformation of a cherished relationship is itself a major source of stress” (p. 584). Thus, low levels of closeness and poor overall quality in the current relationship, as well as the change in relationship closeness in the dyad since dementia onset (e.g. feelings of loss) may contribute to caregiving stress and adverse caregivier outcomes.
Relationship “closeness” in the care dyad is conceptualized as the quality of the emotional bond between the caregiver and care recipient (Whitlatch, et al., 2001). Related concepts, such as relationship quality (Lawrence, Tennstedt, & Assman, 1998; Quinn, Clare, & Woods, 2009), marital closeness (Tower, Kasl, & Moritz, 1997), affectional ties (Bengtson & Roberts, 1991), intimacy (Blieszner & de Vries, 2001), and others, are used in studies of late life dyadic relationships (including caregiver dyadic relationships). Relationship quality includes “feelings of emotional closeness to the older person, having positive sentiment towards the older person, and similarities in values and beliefs” (Lawrence, et al., 1998, p. 150). Affectional ties relates to quality of the interaction in the relationship, expressed via love, respect, trust, appreciation and recognition, also included in models of intergenerational (e.g. parent/child) relationships. In some cases, closeness is linked to a related term, but does not comprise it wholly. Moss and Schwebel (1993) suggest that intimacy consists of: commitment, affective intimacy, cognitive intimacy, physical intimacy, and mutuality. Because closeness is an essential contributor to the components of affective, cognitive, and physical intimacy, closeness is not characterized as its own component (Blieszner & de Vries, 2001). In sum, there is a high degree of overlap in terminology related to emotional connection in a dyadic relationship. The construct of closeness is utilized in the current study, however research on other related concepts is highly relevant.
Relationship Closeness in Care Dyads: Positive Outcomes for Care Recipients and Mixed Findings for Caregivers
Previous studies of closeness in care dyads demonstrate that closer relationships predict positive outcomes for the care recipient, including increased well-being and problem-solving skills (Burgener & Twigg, 2002), more positive adjustment to institutionalization (Whitlatch, et al., 2001), slower cognitive and functional decline (Norton, et al., 2009), and fewer behavioral symptoms (Perren, et al., 2007).
Additionally, several studies report that closer dyad relationships predict more positive caregiver outcomes. Closer spousal relationships are associated with lower caregiver burden (Spaid & Barush, 1994). In mother-daughter dyads, closer caregiver-reported relationships were associated with more satisfaction with the caregiving role (Walker, Shin, & Bird, 1990). Closer prior relationships (before dementia onset) were related to lower levels of caregiver depression (Kramer, 1993; Williamson & Schulz, 1990), lower caregiver burden (Steadman, Tremont, & Davis, 2007; Williamson & Schulz, 1990), lower reactivity to care recipients’ behavioral disturbances, and higher levels of effective caregiver communication (Steadman, et al., 2007), quality of life, and satisfaction with caregiving (Kramer, 1993). Netto, Jenny, and Philip (2009) found that some caregivers feel emotionally closer to the care recipient after dementia onset because caregiving necessitated more frequent interaction in the relationship.
Other studies, however, report that higher relationship closeness predicts adverse psychological caregiver outcomes (Cantor, 1983). Among spousal caregivers, husbands who were emotionally closer to their wives with dementia reported higher levels of depression (Tower, et al., 1997). Contrary to the subset of caregivers described above by Netto and colleagues (2009), some caregivers report a significant loss of closeness or relationship quality when comparing pre- vs. postdementia onset (de Vugt, et al., 2003; Morris, Morris, & Britton, 1988), and loss of dyad intimacy is associated with increased caregiver depression (Morris, et al, 1988). Researchers using longitudinal qualitative interviews found that AD was associated with a decline in intimacy in both spousal and parent-adult offspring care dyads, and relationship loss was linked to caregivers’ feelings of sadness, frustration, and anger (Blieszner & Shifflett, 1990). Lawrence and colleagues (1998) found that for caregivers in high quality dyadic relationships, greater disability in the care recipient predicted greater feelings of caregiver overload.
Although the effect sizes are smaller than those for outcomes related to global and caregiver-specific psychological well-being, there is evidence that caregivers are more at risk for poorer physical health outcomes than non-caregivers, particularly for dementia caregivers (meta-analysis by Pinquart & Sorenson, 2003). A separate meta-analysis supported the same conclusions; caregivers (compared to non-caregivers) were slightly more at risk for 11 health-related outcomes, particularly poorer global health, lower response levels for antibodies, and higher levels of stress hormones (Vitaliano, Zhang, & Scanlan, 2003). There is limited and mixed research on the relationship between care dyad closeness and physical health outcomes for the caregiver. Uchino, Kiecolt-Glaser and Cacioppo (1994) found that higher levels of predementia relationship affection (getting along with/liking each other) predicted better cardiovascular function in caregivers, but higher levels of predementia relationship cohesion (e.g. engaging in activities together, exchanging ideas, working together) predicted poorer cardiovascular function in caregivers. Lyons, Sayer, Archbold, Hornbrook, and Stewart (2007), found that decreases in care dyad mutuality were associated with decreases in caregiver physical health, emphasizing the importance of using time-varying variables in understanding these complex relationships.
In sum, having a closer care dyad relationship seems to be consistently associated with beneficial outcomes for the care recipient, but outcomes for caregivers are mixed. While there is evidence of a link between relationship closeness/quality and caregiver psychological well-being and physical health, we can’t conclude if closeness serves as a risk or protective factor.
The Current Study
The primary goal of this study is to better understand associations between closeness in the care dyad relationship and caregiver psychological well-being and physical health, both cross-sectionally and longitudinally. If closeness predicts consistently positive outcomes for the care recipient, but mixed outcomes for the caregiver, we need more understanding of the associations to inform basic research and clinical practice, which has thus far considered the emotional bond between the care dyad to be a key element of caregiving counseling and clinical intervention (LoboPrabhu, Molinari, & Lomax, 2006). In addition, past research reports that decreases in caregiver and physical health negatively impact caregiver quality of life, as well as non-caregiving roles and relationships (Anderson, Linto, & Stewart-Wynne, 1995), highlighting the importance of predicting level and change over time in caregiver physical health and psychological well-being. We also examine the role of kin relationship (Spruytte, Van Audenhove, Lammertyn, & Storms, 2002), and caregiver gender (Tower, et al., 1997) as moderators, as found in other studies.
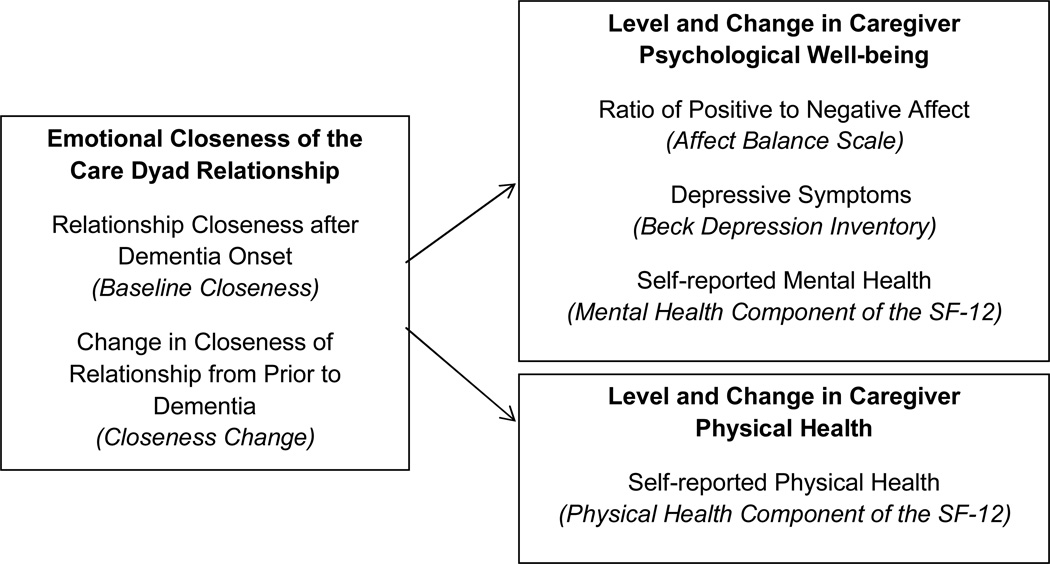
The current study examines the effect of baseline care dyad closeness and change in pre- to postdementia closeness on both baseline levels and change over time in caregivers’ psychological well-being and physical health (see Figure 1). Sin and Lyubomirsky (2009) describe psychological well-being as a global and complex construct that includes one’s affective experience and a cognitive judgment about one’s satisfaction (Schimmack, 2008). In this study, we use three separate indicators to assess this construct. A unique feature of this study is that it utilizes participant dyads from a population-based sample of persons with incident dementia (identified within 2–3 years of dementia onset). Studying persons from the community or population avoids selection biases of clinically ascertained samples that may select for more stressed caregivers. Based on the Stress Process model, we hypothesize that both current closeness level and a loss of closeness in the relationship will predict poorer health and psychological well-being for caregivers (cross-sectionally and longitudinally), with stronger associations for indicators of psychological well-being.
Figure 1.
Constructs, Indicators, and Measures included in the Study
Methods
Participants
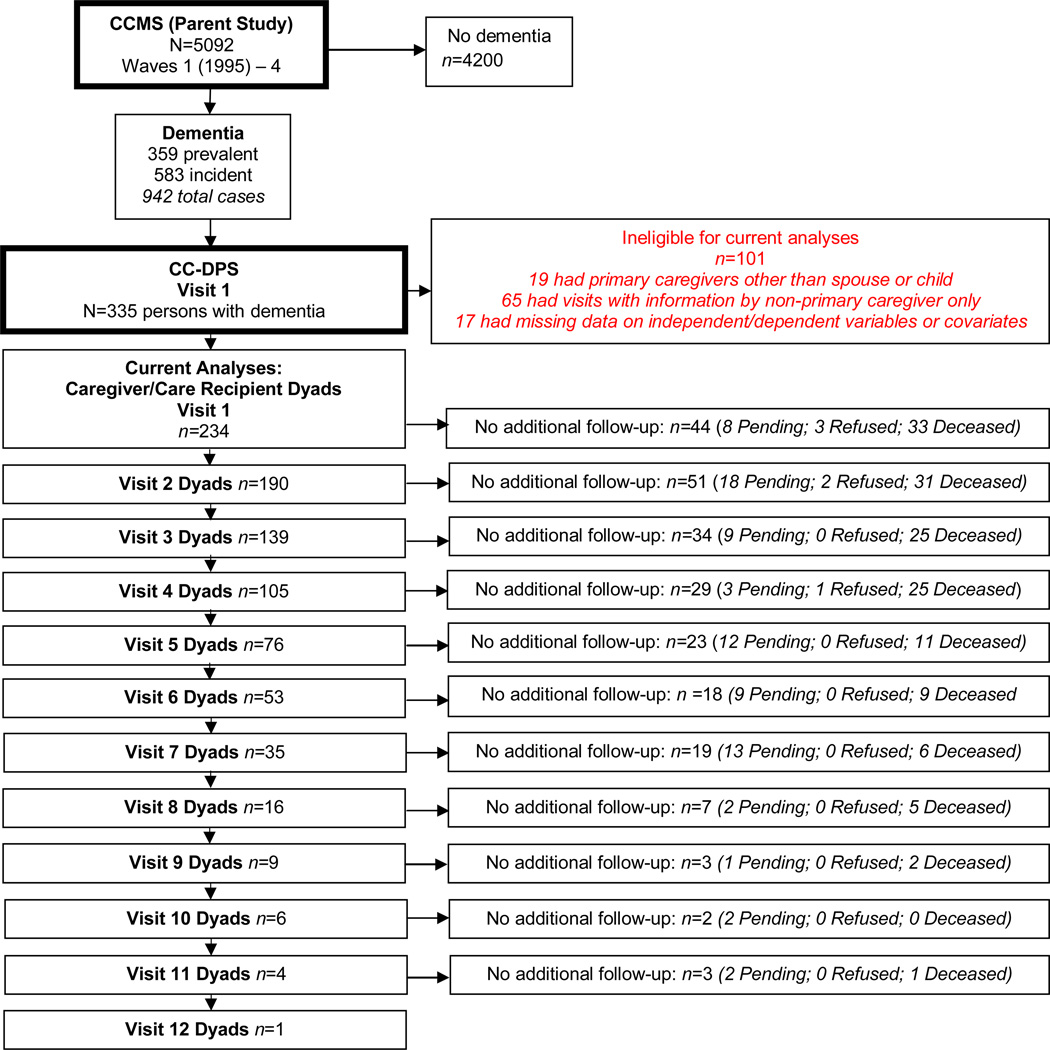
Participant dyads were enrolled in the Cache County Dementia Progression Study (DPS), a follow-up of individuals diagnosed with dementia from the Cache County Memory Study (CCMS). CCMS was a longitudinal epidemiological study of 5,092 older adults in Cache County, Utah, which identified a total of 942 prevalent (existing at baseline) and incident (onset occurring over the course of study) cases of dementia between 1995 and 2007. In CCMS, an expert review panel (neurologists, geriatric psychiatrists, neuropsychologists) diagnosed dementia according to DSM-III-R criteria (see Breitner, et al., 1999). Utilizing criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCSA-ADRDA), a diagnosis of “possible or probable Alzheimer’s disease” was assigned (McKhann, et al., 1984). Of the CCMS participants with dementia, 335 individuals identified from the incidence waves (37.6% of dementia sample) and their caregivers were enrolled in DPS between 2002 and 2009, and were reassessed in their place of residence approximately every 6 months (for details on DPS sample selection, see Tschanz, et al., 2011). For the current study, 234 care dyads were included from DPS. Participants were excluded from the analyses because of non-primary caregiver status (n = 65), caregiver relationship status other than spouse or adult offspring (n=19), or missing data on independent, dependent variables, or covariates (n= 17; see Figure 2).
Figure 2.
Sample Reduction from Cache County Study on Memory in Aging (CCSMA) to Current CC-DPS Sample at each Visit
Note: “Pending” status means that participants would be eligible for follow-up assessment but have not been re-visited because DPS data collection ended before the subsequent visit.
Measures
Assessments were conducted by a trained research nurse and neuropsychological technician, and written informed consent was obtained for each assessment (see Tschanz et al., 2011). Study procedures were approved by the Institutional Review Boards of Utah State University, and the Johns Hopkins University.
Dependent Variables
The Affect Balance Scale (ABS; Bradburn, 1969; Coleman, Philip, & Mulle, 1995) is a 10-item self-report assessment of whether 5 negative emotions and 5 positive emotions were experienced in the past week (1=yes; 0=no). In the ABS index, a higher score indicates a greater proportion of positive psychological well-being. Its validity and reliability are well established (Bradburn, 1969; Diener, 1984; McDowell & Newell, 1987) with test-retest reliability of 0.76, and internal consistency of Positive Affect items ranging between 0.55–0.73, and of Negative Affect items ranging from 0.61–0.73 (Schiaffino, 2003); in the current sample Cronbach α of the whole index = 0.639. We estimated models using the single index described above, as well as the positive and negative scores separately. Results did not differ in models using items individually or combined into the single index, so the single index score is reported for all final models.
Caregiver depressive symptoms were measured via the 21-item self-report Beck Depression Inventory II (BDI; Beck, Rush, Shaw, & Emery, 1979). Each item uses a 4-point (0 to 3) Likert scale to assess the presence of symptoms over the past 2 weeks. Higher values on summed score indicate worse depressive symptoms such as guilt, sadness, crying, and social withdrawal. The scale has demonstrated appropriate reliability and validity in mixed-age samples (see Hunsley & Mash, 2008), and specifically in older adult samples (Gallagher, Nies, & Thompson, 1982). Internal consistency in the current sample was high (Cronbach α = 0.869).
Caregiver physical and mental health over the previous four weeks was assessed using the Short-Form Health Survey, SF-12 (a short version of the SF-36; Ware, Kosinski, & Keller, 1996). The SF-12 includes a Physical Component, SF-12 PC, and Mental Component, SF-12 MC, assessing the extent to which normal roles are impacted by physical or emotional capabilities, respectively. Sample items from the SF-12 PC include “How much does your health limit you in the following activities: Climbing several flights of stairs” (not limited at all, limited a little, limited a lot); “In general would you say your health is” (excellent, very good, good, fair, poor). Sample items from the SF-12 MC include “How much of the time in the past 4 weeks did you feel calm and peaceful” (all of the time, most of the time, good bit of the time, some of the time, a little of the time, none of the time); “During the past 4 weeks have you accomplished less than you would like with your work or other regular daily activities as a result of any emotional problems, such as feeling depressed or anxious” (yes, no). SF-12 items are recoded according to scale instructions, and combined into the SF-PC and SF-MC. Subscales range from 0–100, where 50 represents an average, or normed score, and higher scores indicate better health. The SF-MC and SF-PC have been used widely in a variety of clinical and research settings, demonstrating strong psychometric properties (Lee, Browne, & Villanueva, 2008; Ware, et al., 1996).
Independent Variable
Relationship Closeness was assessed at baseline via the Relationship Closeness Scale, a six-item instrument developed by Noelker (1996) and Whitlach, et al. (2001). The measure assesses caregiver agreement with six statements about their current relationship with the care recipient, with responses coded using a 4-point Likert scale. Items in the scale are (a) “My relative always understands what I value in life,” (b) “My relationship with my relative has always been close,” (c) “My relative always makes me feel that whatever I do for him/her is not enough (reverse coded),” (d) “My relative always makes me feel like a special person,” (e) “My relative is often critical of me (reverse coded)”, and (f) “My relative and I can always discuss things together”. The scale has a reported alpha reliability of 0.90 (Whitlatch, et al., 2001), and in the current sample Cronbach α = 0.859. Responses are summed such that higher scores indicate closer dyad relationships. Caregivers are also asked about their relationship with the care recipient prior to the onset of dementia using the same items. Our Closeness Change score was created by subtracting predementia closeness scores from current closeness scores, both assessed at baseline, such that negative scores indicate a greater loss of closeness prior to the onset of dementia.
Covariates
Demographic characteristics included in the analyses were: caregivers’ age, years of education, gender, and kin relationship to care recipient, as well as care recipients’ age and gender. Caregiver gender and kin relationship combinations were defined as: female spouse, male spouse, female adult offspring, and male adult offspring. Models were run including 19 individuals reporting “other” kin relationships, however findings were robust, so these were excluded in final models to allow more parsimonious interpretation of kin relationship variables. Caregiver comorbidity was captured as the number of endorsed health conditions out of 11. Dementia type was dichotomized into Possible/Probable AD vs. all other dementias.
Duration of dementia was measured as years from diagnosis to initial assessment in DPS baseline. The frequency*severity products were summed across 10 behavioral domains from the Neuropsychiatric Inventory (NPI; Cummings, et al., 1994) to assess total neuropsychiatric symptoms (delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/Indifference, disinhibition, irritability/lability, and aberrant motor behavior). Higher scores indicate more severe behavioral symptoms. The functional status of care recipients was assessed by summing nurse ratings on memory, orientation, problem solving, community involvement, and abilities in home and personal affairs producing the “sum of boxes” score on the Clinical Dementia Rating scale (CDR; Hughes, et al., 1982).
Analyses
To examine associations between predictor variables and baseline level and longitudinal change in the four caregiver outcomes, we conducted linear mixed models. This method accounts for correlations between repeated measures made on each participant, while allowing for imbalance in the number of available measurement visits per participant. Linear and quadratic time effects were initially tested, with time2 term removed in models reported herein, as it was non-significant. The first unadjusted model (no covariates) for each outcome variable included time, baseline closeness, and their interaction. The second base model included time, closeness change, and their interaction. Models with significant interaction terms were re-run with covariates included. Terms were retained in the model if the individual term had an associated Wald statistic with p<0.05 or if the likelihood ratio (LR) chi-square test of models with and without the new terms yielded a p<0.05. Analyses were conducted using SAS Version 9.2.
Results
Table 1 displays sample characteristics for dyads at baseline. Three quarters of the caregivers were female and the most common caregiver type (42%) was adult daughter. Caregivers were observed between 0.8 – 14.8 years after dementia onset. Nearly two-thirds of care recipients had a diagnosis of AD (62%), 52% were female, with average age of 82 years (SD=6.22).
Table 1.
Descriptive Characteristics of Care Dyad Participants at Baseline
| Caregiver | |
|---|---|
| Kin relationship and Gender: | |
| Female adult offspring N (%) | 98 (41.88) |
| Female spouse N (%) | 80 (34.19) |
| Male adult offspring N (%) | 22 (9.40) |
| Male spouse N (%) | 34 (14.53) |
| Age M (SD) | 69.47 (14.0) |
| Education in years M (SD) | 14.2 (2.37) |
| Number of medical conditions M (SD) | 1.91 (1.45) |
| Number of follow-up visits | |
| All participants M (SD) | 2.35 (2.31) |
| Participants with more than one visit | 3.13 (2.16) |
| Relationship Closeness at Baseline M (SD) | 18.9 (4.04) |
| Closeness Change M (SD) | −0.60 (3.02) |
| Affect Balance M (SD) | 7.59 (1.97) |
| Beck Depression Inventory M (SD) | 8.07 (9.03) |
| SF-12 Mental Health Component M (SD) | 50.50 (9.18) |
| SF-12 Physical Health Component M (SD) | 49.89 (10.72) |
| Care recipient | |
| Male N (%) | 112 (48) |
| Female N (%) | 122 (52) |
| Age M (SD) | 82.0 (6.22) |
| Possible/Probable Alzheimer’s disease diagnosis N (%) | 144 (61.54) |
| Dementia duration in years M (SD) | 3.29 (1.80) |
Note: Caregivers were observed between 0.8 – 14.8 years after dementia onset. Negative scores in Closeness Change indicate a greater loss of closeness from predementia levels to postdementia levels
For each outcome, unadjusted models tested for significant change over time as a function of baseline closeness or Closeness Change (Table 2). Results from the models testing psychological well-being outcomes found that higher baseline closeness predicted higher baseline Affect Balance (ABS), as well as significant decreases in ABS over time. Loss of closeness (negative scores in Closeness Change) predicted lower baseline ABS, and increases in ABS over time. Caregivers reporting higher baseline closeness reported significantly lower baseline BDI, but closeness was not related to change in BDI over time, and Closeness Change was not related to baseline level or rate of change in depressive symptoms. Higher baseline closeness predicted higher baseline SF-12 MC scores but significant decreases in SF-12 MC over time. For results from the model of physical health, baseline closeness was not significantly associated with baseline level or rate of change in SF-12 PC, however loss of closeness predicted decreases in SF-12 PC over time (although it was not associated with baseline SF-12 PC). Because change over time in caregiver outcomes was of primary interest in the current analyses, all models with a significant Baseline Closeness* time or Closeness Change*time interaction term were carried forward into additional models with covariates included.
Table 2.
Base Mixed Models for Caregiver Outcomes without Covariates
| Estimate | SE | T-statistic | p-value | |
|---|---|---|---|---|
| Model 1: Affect Balance | ||||
| Intercept | 4.40 | 0.60 | 7.35 | <0.01 * |
| Time | 0.84 | 0.27 | 3.10 | <0.01 * |
| Baseline Closeness | 0.16 | 0.03 | 5.25 | <0.01 * |
| Baseline Closeness*Time | −0.05 | 0.01 | −3.34 | <0.01 * |
| Model 2: Affect Balance | ||||
| Intercept | 7.55 | 0.13 | 57.76 | <0.01 * |
| Time | −0.10 | 0.06 | −1.79 | 0.08 |
| Closeness Change | 0.09 | 0.04 | 2.33 | 0.02 * |
| Change in Closeness* Time | −0.05 | 0.02 | −2.65 | <0.01 * |
| Model 3: Beck Depression Inventory | ||||
| Intercept | 15.04 | 2.81 | 5.35 | <0.01 * |
| Time | −1.67 | 1.31 | −1.27 | 0.20 |
| Baseline Closeness | −0.35 | 0.15 | −2.4 | 0.02 * |
| Baseline Closeness*Time | 0.07 | 0.07 | 1.04 | 0.30 |
| Model 4: Beck Depression Inventory | ||||
| Intercept | 8.49 | 0.63 | 13.51 | <0.01 * |
| Time | −0.35 | 0.30 | −1.17 | 0.24 |
| Closeness Change | 0.05 | 0.20 | 0.27 | 0.79 |
| Closeness Change * Time | −0.03 | 0.10 | −0.28 | 0.78 |
| Model 5: SF-12: Mental Health Component | ||||
| Intercept | 38.00 | 2.59 | 14.67 | <0.01 * |
| Time | 3.16 | 1.40 | 2.24 | 0.02 * |
| Baseline Closeness | 0.68 | 0.13 | 5.10 | <0.01 * |
| Baseline Closeness*Time | −0.18 | 0.07 | −2.51 | 0.01 * |
| Model 6: SF-12: Mental Health Component | ||||
| Intercept | 51.07 | 0.58 | 87.74 | <0.01 * |
| Time | −0.53 | 0.30 | −1.79 | 0.08 |
| Closeness Change | 0.29 | 0.19 | 1.57 | 0.11 |
| Closeness Change * Time | −0.30 | 0.11 | −2.80 | <0.01 * |
| Model 7: SF-12: Physical Health Component | ||||
| Intercept | 48.13 | 3.32 | 14.49 | <0.01 * |
| Time | −2.03 | 1.28 | −1.59 | 0.11 |
| Baseline Closeness | 0.03 | 0.17 | 0.19 | 0.85 |
| Baseline Closeness*Time | 0.09 | 0.07 | 1.42 | 0.16 |
| Model 8: SF-12: Physical Health Component | ||||
| Intercept | 49.00 | 0.71 | 68.55 | <0.01 * |
| Time | −0.11 | 0.25 | −0.44 | 0.66 |
| Closeness Change | 0.06 | 0.23 | 0.26 | 0.79 |
| Closeness Change * Time | 0.22 | 0.10 | 2.18 | 0.03 * |
Note: Negative scores in Closeness Change indicate a greater loss of closeness from predementia levels to postdementia levels.
indicates statistical significance at a level of p < 0.05.
Baseline Closeness and Closeness Change Predict Change in Caregiver Affect over Time
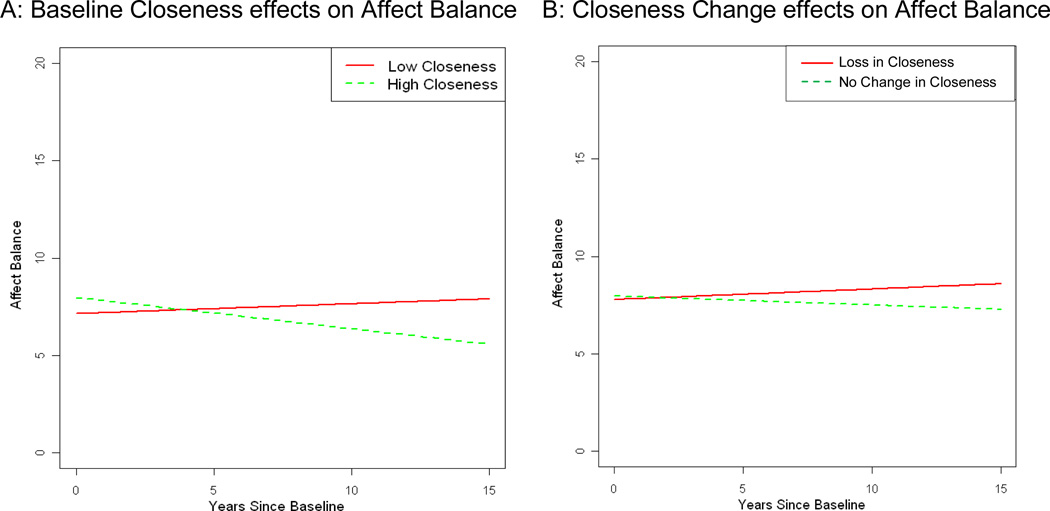
For the final models predicting caregiver affect (ABS; Table 3), model 1 suggests that having fewer caregiver health conditions, and having AD diagnoses and lower NPI scores in the care recipients predicted higher baseline ABS. There was a significant interaction with closeness for male adult offspring; male offspring with closer relationships had higher baseline ABS than male spouse caregivers with high closeness. Having a greater number of baseline caregiver health conditions also predicted increases in ABS over time. For the main associations of interest, higher baseline closeness was not associated with higher baseline ABS after controlling for covariates, but baseline closeness significantly predicted decreases in ABS over time.
Table 3.
Effect of Baseline Closeness and Closeness Change on Trajectory of Affect Balance: Results of the Final Model
| Model Term | Estimate | SE | T-statistic | p-value |
|---|---|---|---|---|
| Model 1: Effect of Baseline Closeness | ||||
| Intercept | 7.27 | 1.65 | 4.40 | <0.01* |
| Time | 0.56 | 0.27 | 2.11 | 0.04* |
| Baseline Closeness | 0.02 | 0.08 | 0.24 | 0.81 |
| Time*Baseline Closeness | −0.04 | 0.01 | −3.16 | <0.01* |
| Alzheimer’s Diagnosis | 0.43 | 0.21 | 2.05 | 0.04* |
| Female Adult offspring Caregiver | −2.06 | 1.74 | −1.18 | 0.24 |
| Female Spouse Caregiver | 0.28 | 1.87 | 0.15 | 0.88 |
| Male Adult offspring Caregiver | −5.56 | 2.89 | −1.92 | 0.06 |
| Male Spouse Caregiver | 0.00 | . | . | . |
| Baseline Closeness*Female Adult offspring Caregiver | 0.15 | 0.09 | 1.67 | 0.10 |
| Baseline Closeness*Female Spouse Caregiver | 0.02 | 0.09 | 0.21 | 0.84 |
| Baseline Closeness*Male Adult offspring Caregiver | 0.30 | 0.14 | 2.12 | 0.04* |
| Baseline Closeness*Male spouse Caregiver | 0.00 | . | . | . |
| Number of Health Conditions | −0.39 | 0.07 | −5.78 | <0.01* |
| Time* Number of Health Conditions | 0.10 | 0.04 | 2.83 | 0.01* |
| Behavioral Symptoms of Care Recipient (NPI) | −0.03 | 0.01 | −3.67 | <0.01* |
| Model 2: Effect of Closeness Change | ||||
| Intercept | 8.02 | 0.22 | 36.92 | <0.01* |
| Time | −0.04 | 0.06 | −0.77 | 0.44 |
| Closeness Change | 0.00 | 0.06 | −0.03 | 0.98 |
| Time * Closeness Change | −0.05 | 0.02 | −2.47 | 0.01* |
| Alzheimer’s Diagnosis | 0.57 | 0.21 | 2.69 | 0.01* |
| Number of Health Conditions | −0.28 | 0.06 | −4.97 | <0.01* |
| Time* Number of Health Conditions | 0.07 | 0.02 | 3.07 | <0.01* |
| Behavioral Symptoms of Care Recipient (NPI) | −0.04 | 0.01 | −4.70 | <0.01* |
| Time* Behavioral Symptoms of Care Recipient (NPI) | −0.01 | 0.00 | −2.93 | <0.01* |
Note: Male Spouse is the reference group for the categorical kin relationship variable.
indicates statistical significance at a level of p < 0.05.
Model two supported similar outcomes for covariates as Model 1, however the closeness by male adult offspring interaction was not significant in model 2, and a significant time X NPI interaction emerged, suggesting that in model 2, greater behavioral symptoms predicted decreases in ABS over time. For the main associations of interest, Closeness Change was not associated with baseline ABS after controlling for covariates, but a loss of closeness before dementia onset to postdementia predicted increases in ABS over time.
Figure 3 graphically represents A) the average change over time for low levels of baseline closeness (estimated for individuals with a baseline closeness score of 17 – the 25th percentile score) and high closeness (score of 22 – the 75th percentile score), and B) the average change over time for caregivers reporting negative Closeness Change (score of −2 in Closeness Change – the 25th percentile score) and no Closeness Change (score of 0 – the 75th percentile score).
Figure 3.
Affect Balance: A) Closer caregivers compared to less close caregivers; and B) Caregivers reporting loss in closeness compared to those reporting no change in closeness
Note: Lines represent change for prototypical levels (25th and 75th percentiles) of A) baseline closeness and B) change in closeness from predementia to baseline (postdementia) closeness. Loss in Closeness (25th percentile) indicates care dyads decreased in closeness from predementia to postdementia; No Change in Closeness (75th percentile) indicates that there was no reported change in closeness scores from predementia to postdementia
Baseline Closeness and Closeness Change Predict Change in SF-12 Mental Health Component over Time
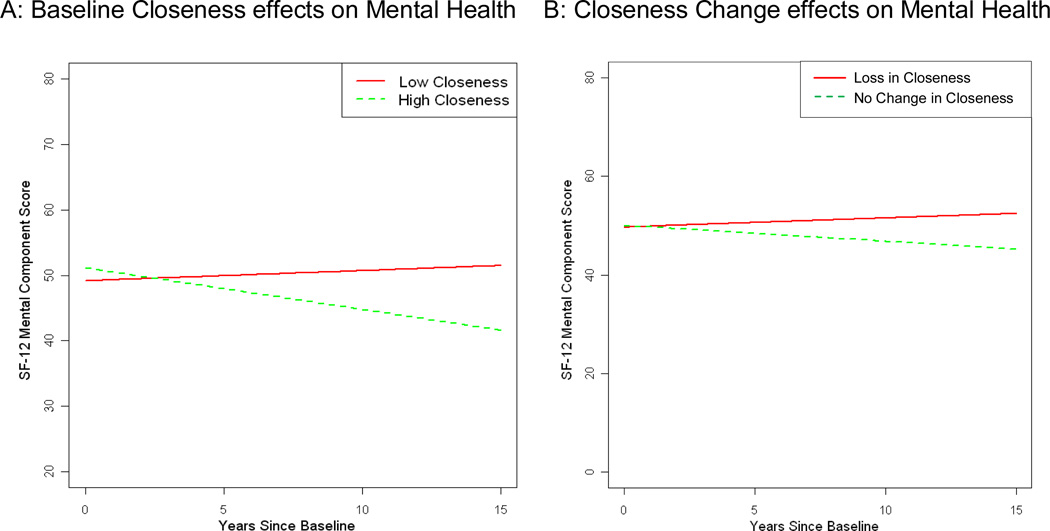
Table 4 displays the final models for SF-12 MC. Model 1 suggests that female adult offspring had lower baseline SF-12 MC scores than male spouses, and fewer caregiver health conditions predicted higher baseline SF-12 MC. Longitudinal declines in SF-12 MC were predicted by higher NPI, but longitudinal increases in SF-12 MC were predicted by a greater number of caregiver health conditions at baseline. For the main outcomes of interest, a closer care dyad relationship at baseline predicted higher baseline SF-12 MC, but significant decreases in SF-12 MC scores over time (Figure 4). In model 2, the same covariate relationships emerged as in model 1, and for the outcomes of interest, Closeness Change was not associated with baseline SF-12 MC, but loss of closeness predicted increases in SF-12 MC over time (see Figure 4).
Table 4.
Effect of Baseline Closeness and Closeness Change on Trajectory of SF-12 Mental Health Component: Results of the Final Model
| Model Term | Estimate | SE | T-Statistic | p-value |
|---|---|---|---|---|
| Model 1: Effect of Baseline Closeness | ||||
| Intercept | 50.20 | 2.98 | 16.83 | <0.01* |
| Time | 1.53 | 1.32 | 1.16 | 0.25 |
| Baseline Closeness | 0.42 | 0.13 | 3.24 | <0.01* |
| Time*Baseline Closeness | −0.16 | 0.07 | −2.40 | 0.02* |
| Female Adult offspring | −3.80 | 1.39 | −2.73 | 0.01* |
| Female Spouse | −0.97 | 1.40 | −0.69 | 0.49 |
| Male Adult offspring | −3.56 | 1.93 | −1.84 | 0.07 |
| Male Spouse | 0.00 | . | . | . |
| Number of Health Conditions | −1.90 | 0.31 | −6.10 | <0.01* |
| Time* Number of Health Conditions | 0.67 | 0.18 | 3.76 | <0.01* |
| Time* Behavioral Symptoms of Care Recipient (NPI) | −0.16 | 0.03 | −4.59 | <0.01* |
| Model 2: Effect of Closeness Change | ||||
| Intercept | 58.86 | 1.39 | 42.45 | <0.01* |
| Time | −1.68 | 0.46 | −3.67 | <0.01* |
| Closeness Change | 0.18 | 0.17 | 1.05 | 0.29 |
| Time * Closeness Change | −0.25 | 0.10 | −2.50 | 0.01* |
| Female Adult offspring | −4.17 | 1.42 | −2.95 | <0.01* |
| Female Spouse | −1.33 | 1.42 | −0.94 | 0.35 |
| Male Adult offspring | −3.56 | 1.97 | −1.80 | 0.07 |
| Male Spouse | 0.00 | . | . | . |
| Number of Health Conditions | −2.01 | 0.32 | −6.30 | <0.01* |
| Time* Number of Health Conditions | 0.69 | 0.19 | 3.74 | <0.01* |
| Time* Behavioral Symptoms of Care Recipient (NPI) | −0.18 | 0.03 | −5.21 | <0.01* |
indicates statistical significance at a level of p < 0.05.
Figure 4.
SF-12 Mental Health Component: A) Closer caregivers compared to less close caregivers; and B) Caregivers reporting loss in closeness compared to those reporting no change in closeness
Note: Lines represent change for prototypical levels (25th and 75th percentiles) of A) baseline closeness and B) change in closeness from predementia to baseline (postdementia) closeness. Loss in Closeness (25th percentile) indicates care dyads decreased in closeness from predementia to postdementia; No Change in Closeness (75th percentile) indicates that there was no reported change in closeness scores from predementia to postdementia
Closeness Change Predicts Change in SF-12 Physical Health Component over Time
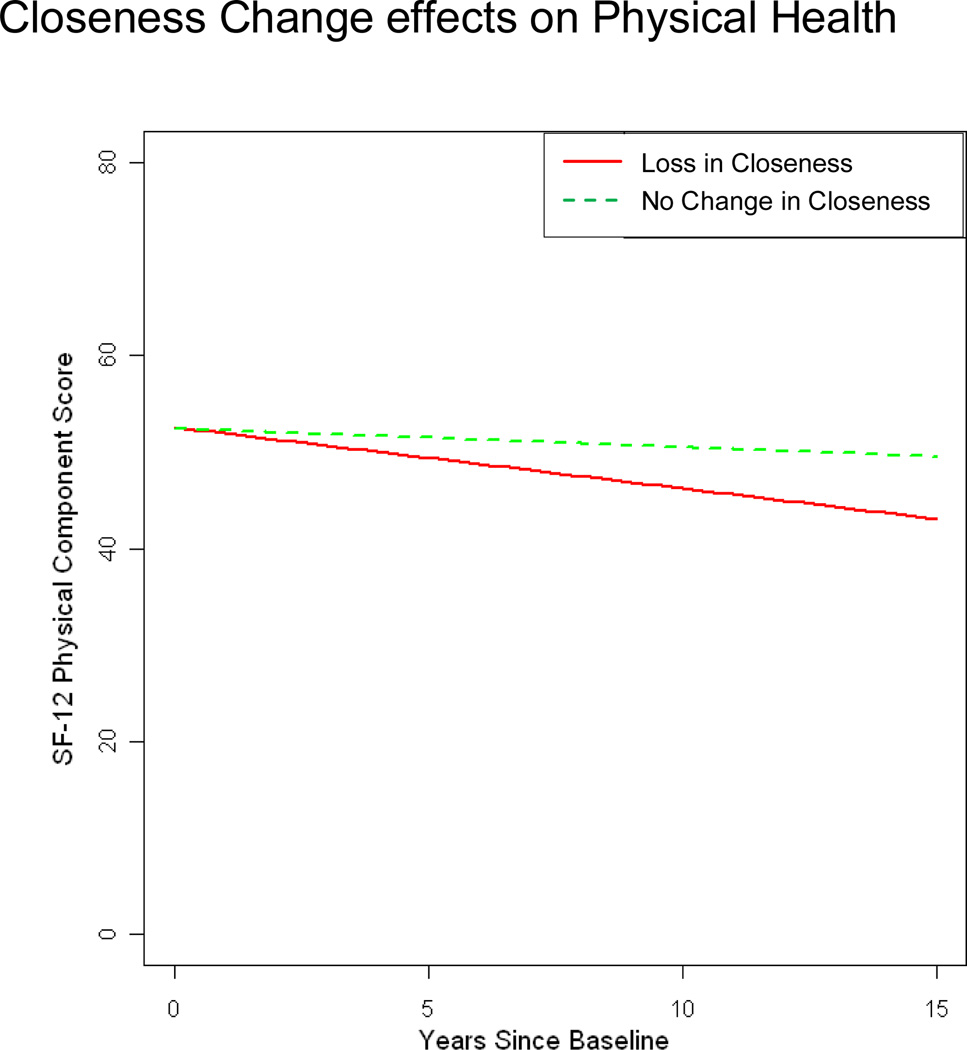
Covariate results from the final model suggest that female adult offspring had significantly better SF-12 PC scores at baseline compared to male spouse caregivers, and having a higher number of health conditions at baseline predicted lower baseline SF-12 PC (Table 5). Closeness Change was not associated with baseline SF-12 PC, but greater losses in closeness predicted poorer SF-12 PC over time (Figure 5).
Table 5.
Effect of Closeness Change on Trajectory of SF-12 Physical Component: Results of the Final Model
| Model Term | Estimate | SE | T-Statistic | p-value |
|---|---|---|---|---|
| Intercept | 49.38 | 1.64 | 30.07 | 0.00* |
| Time | −0.19 | 0.26 | −0.74 | 0.46 |
| Closeness Change | −0.06 | 0.20 | −0.29 | 0.77 |
| Time * Closeness Change | 0.22 | 0.10 | 2.19 | 0.03* |
| Female Adult offspring | 6.26 | 1.77 | 3.54 | 0.00* |
| Female Spouse | 0.19 | 1.80 | 0.10 | 0.92 |
| Male Adult offspring | 3.46 | 2.47 | 1.40 | 0.16 |
| Male Spouse | 0.00 | . | . | . |
| Number of Health Conditions | −1.81 | 0.30 | −6.12 | 0.00* |
indicates statistical significance at a level of p < 0.05.
Figure 5.
SF-12 Physical Health Component: Caregivers reporting loss in closeness compared to those reporting no change in closeness
Note: Lines represent change for prototypical levels (25th and 75th percentiles) of change in closeness from predementia to baseline (postdementia) closeness. Loss in Closeness (25th percentile) indicates care dyads decreased in closeness from predementia to postdementia; No Change in Closeness (75th percentile) indicates that there was no reported change in closeness scores from predementia to postdementia
Discussion
In this population-based sample of caregivers and care recipients with dementia, the association between care dyad relationship closeness and caregiver outcomes differed between cross-sectional and longitudinal analyses, and between psychological well-being and physical health outcomes. After controlling for covariates, cross-sectional results suggest that higher levels of baseline closeness were significantly associated with less depression (BDI) and better mental health ratings (SF-12 MC) but not with caregiver affect (ABS) or with caregivers’ physical health scores (SF-12 PC). In contrast, longitudinal findings suggest that higher baseline closeness is associated with worse outcomes; higher closeness predicts declines over time in caregiver ABS and SF-12 MC. To add to the complexity, after controlling for covariates, Closeness Change was not associated with baseline levels of any of caregiver measures included in this study, yet loss of closeness was associated with increases in psychological wellbeing (ABS, SF-12 MC), and declines in physical health (SF-12 PC). Although these mixed outcomes do not allow us to conclude that closer dyadic relationships serve as consistent risk or protective factors for caregivers, our findings reflect prior conclusions that closeness predicts both beneficial (Spaid & Barush, 1994; Walker, Shin, & Bird, 1990; Williamson & Schulz, 1990) and adverse (Cantor, 1983; Tower, et al., 1997; Blieszner & Shifflett, 1990) caregiver outcomes.
The current study offers two important suggestions for better understanding these complex associations. First, our study supports that both current closeness (after dementia onset) and changes in closeness from the predementia relationship are important to assess when predicting caregiver outcomes. Second, our findings suggest that taking a purely cross-sectional view of these associations is misleading since closeness is related to better caregiver outcomes cross-sectionally, but worse outcomes longitudinally. Our results are only partially consistent with the Stress Process model, which describes relational deprivation with a loved one as having negative consequences for caregiver stress and well-being (Pearlin, et al.,1990). Our finding that higher levels of current closeness predicted declines in affect and mental health for the caregiver, and that a greater loss in the relationship (from pre- to postdementia) predicted poorer physical health over time together support that poorer relationship quality and loss in the relationship may serve as sources of stress for caregivers, negatively impacting their physical health and psychological well-being. However, greater loss in closeness from pre- to postdementia predicted improvements in affect and mental well-being over time, which does not support the view that relationship loss is a stressor. These findings suggest that perhaps a withdrawal from the emotional bonds in a relationship (detachment) may protect the caregiver from deteriorating psychological well-being. This suggestion needs to be explored further, but it is supported by caregiving research which proposes that there is a loss of intimacy over the course of caregiving where the caregivers learn to “become strangers” with the care recipient as an adaptive mechanism (Wuest, Ericson, & Stern, 1994). Chesla and colleagues (1994) reported that for some caregivers, increasing closeness is too emotionally difficult, and even in dyads where dementia causes the relationship to become discontinuous and detached, caregivers still focused on providing high quality care, suggesting that if closeness is lost, the caregiver may be protected emotionally but the care recipient does not necessarily face a poorer care environment. Future studies may wish to test whether loss of closeness is related to greater withdrawal, and whether this coping strategy is predictive of better or worse outcomes for caregivers and care recipients.
One question that arises from the current analysis is why changes over time in ABS and SF-12 MC were associated with closeness, but change in BDI was not. We suspect there may be two possible explanations for this finding. First, because this is a population-based study, caregivers had relatively low levels of depressive symptoms at baseline (mean BDI = 8.07). Second, a measure of Affect Balance may be more sensitive to change in non-depressed samples – that is, it may be easier to detect “less positive mood” than “more depressive symptoms.”
Research on the role of relationship quality characteristics and physical health outcomes for caregivers is scarce, however our findings fit with some of the available research in this area (Uchino, et al., 1994; Lyons, et al., 2007). The finding that Closeness Change was a significant predictor of SF-12 PC (and baseline closeness was not predictive) suggests that low levels of prior closeness may be driving this association. The presence of a positive Closeness Change score in an individual suggests that his or her report of prior closeness might be particularly low, whereas negative Closeness Change scores may be driven by high levels of predementia closeness. The study reporting that relationship affection was positively associated with physical health outcomes (Uchino, et al., 1994) utilized prior closeness as their independent variable. Similarly, Lyons and colleagues (2007) reported positive associations between changes in relationship mutuality and changes in caregivers’ physical health. Therefore the associations between closeness and physical health outcomes may be somewhat dependent on utilizing measures of closeness prior to dementia onset, and or the extent to which that relationship closeness changes over time.
Study Strengths and Limitations
Caregiver research is frequently based on convenience or clinical samples, and less often utilizes population-based samples (see Castro, et al., 2007; Cochrane, Goering, & Rogers, 1997). A strength of the current study is that care dyads were selected from a population-based sample of individuals with incident dementia and their caregivers, assessed at frequent intervals (every 6 months). Participation rates were high, with small rates of drop-out due to refusal. Despite these strengths, a limitation is that the sample consisted of mostly White participants from northern Utah, and results may not generalize to more ethnically diverse populations. A review by Dillworth-Anderson and Gibson (2002) discussed differences across various ethnic groups in regards to emotional and relational aspects of caregiving. Cultural expectations vary regarding family relationships, filial piety, and relationship reciprocity, suggesting that dyadic closeness may serve different, more important, or less important roles in different cultures. For example, in cultures where caregivers and care recipients have lived in extended households for all of adulthood, closer emotional bonds may have existed prior to dementia onset and feelings of emotional loss after dementia onset may portend adverse emotional outcomes for caregivers. Studies of care dyad closeness using cross-cultural caregiving samples are needed.
An additional strength of the study is the measurement of closeness at baseline and closeness change over time. Ablitt, Jones, and Muer (2009) reviewed relationship factors in dementia and found support for the idea that care dyad relationships evolved as the illness progressed. Hellström, Nolan, and Lundh (2007) suggest that change in care dyad relationships may be more iterative than linear in nature. While assessing change in our study is an improvement over past studies of closeness, a limitation of our Closeness Change measure is that predementia closeness reports are retrospective. While this is a common way to assess relationship closeness prior to dementia onset, there might be less bias with actual measurements obtained prior to the onset of dementia. Second, one of the items in the scale is “My relationship with my relative has always been close”. We recognize that this language creates some measurement “noise” when comparing current closeness and prior closeness, as the current closeness measure will be influenced by historical reference of whether or not the relationship has “always been close”. The use of this item in a current closeness measure implies an inherent stability in the relationship closeness construct such that always being close contributes to current closeness. Alternatively we recognize that relationships are not stagnant, and while under normal circumstances dyadic relations can be considered dynamic, dementia progression may make care dyad relationships even more susceptible to change. Using a scale of closeness that doesn’t include past relationship status in current relationship assessment should be further explored. Likewise we need to recognize that studies using one assessment of “current closeness” may not adequately capture the more complex and dynamic dyadic relationship.
Recommendations for Future Research
Future investigations will expand upon the present study to examine how relationship closeness changes during the course of dementia, and how this relates to caregiver outcomes. We also wish to explore specific aspects of relationship closeness that impact caregiver outcomes. For example, Norton and colleagues (2009) reported that one item (the extent to which caregivers felt appreciated) appeared to drive the association between closer relationships and slower decline in the care recipient. It is possible that feelings of reciprocity in the dyadic relationship may be a particularly important element of closeness in associations between closeness and caregiver outcomes. Exchange theory suggests that lack of reciprocity from the care recipient would predict poorer outcomes for caregivers, yet exchange theory is not consistently supported in studies of late-life caregiving (Dwyer, Lee, Jankowski, 1994). Dwyer and colleagues advise that researchers may need to distinguish between short term reciprocity where caregivers currently receive less than they give to the relationship, vs. reciprocity over the lifetime where caregivers perceive that what they give to the relationship balances with what the care recipient gave to them at prior points in time. Finally, we propose that future studies examine the dynamics of closeness and psychological well-being and physical health to observe whether closeness has differential outcomes that fluctuate over time. It is our hope that research can maximize longitudinal studies involving the caregiver and care recipient to identify an optimal balance of relationship closeness, or certain behaviors associated with closer relationships that lead to maximizing positive outcomes for both members of the dyad.
Acknowledgements
The authors are indebted to Dr. Ronald Munger for his unqualified support of the DPS. We also acknowledge the contributions of the following individuals whose activities have helped to ensure the success of the project: John C.S. Breitner, M.D., M.P.H., Cara Brewer, B.A., Tony Calvert, R.N., B.A., Michelle Carlson, Ph.D., Kimberly Graham, B.A., Robert C. Green, M.D., M.P.H., Hochang Ben Lee, M.D., Jeanne-Marie Leoutsakos, Ph.D., Carol Leslie, M.S., Lawrence S. Mayer, Ph.D., Michelle M. Mielke, Ph.D.,Chiadi U. Onyike, M.D., Roxane Pfister, M.S., Georgiann Sanborn, M.S., Nancy Sassano, Ph.D., Sarah Schwartz, M.S, Ingmar Skoog, M.D., Martin Steinberg, M.D., Katherine Treiber, Ph.D., Yorghos Tripodis, Ph.D Kathleen A. Welsh-Bohmer, Ph.D., Heidi Wengreen, Ph.D.,RD, James Wyatt, and Peter P. Zandi, Ph.D., M.P.H. Finally, we thank the participants and their families for their participation and support.
Funded by NIA grants R01AG21136, R01AG11380.
Footnotes
Presented in preliminary form at the annual meeting of the Gerontological Society of America, Nov. 2010.
References
- Ablitt A, Jones GV, Muers J. Living with dementia: A systematic review of the influence of relationship factors. Aging & Mental Health. 2009;13(4):497–511. doi: 10.1080/13607860902774436. [DOI] [PubMed] [Google Scholar]
- Anderson CS, Linto J, Stewart-Wynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke. 1995;26:843–849. doi: 10.1161/01.str.26.5.843. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford; 1979. [Google Scholar]
- Bengtson VL, Roberts EL. Intergenerational solidarity in aging families: An example of formal theory construction. Journal of Marriage and the Family. 1991;53:856–870. [Google Scholar]
- Blieszner R, de Vries B. Perspectives on intimacy. Generations. 2001;25(2):7–8. [Google Scholar]
- Blieszner R, Shifflett PA. The effects of Alzheimer's disease on close relationships between patients and caregivers. Family Relations. 1990;39:57–62. [Google Scholar]
- Bradburn NM. The structure of psychological well-being. Chicago, Ill: Aldine; 1969. [Google Scholar]
- Breitner JCS, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, et al. APOE-ε4 count predicts age when prevalence of AD increases, then declines: The Cache County Study. Neurology. 1999;53:321–336. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Burgener S, Twigg P. Relationships among caregiver factors and quality of life in care recipients with irreversible dementia. Alzheimer Disease and Associated Disorders. 2002;16:88–102. doi: 10.1097/00002093-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Cantor MH. Strain among caregivers: A study of experience in the United States. The Gerontologist. 1983;23:597–604. doi: 10.1093/geront/23.6.597. [DOI] [PubMed] [Google Scholar]
- Castro CM, King AC, Housemann R, Bacak SJ, McMullen KM, Brownson RC. Rural family caregivers and health behaviors: Results from an epidemiologic survey. Journal of Aging and Health. 2007;19:87–105. doi: 10.1177/0898264306296870. [DOI] [PubMed] [Google Scholar]
- Chesla C, Martinson I, Muwaswes M. Continuities and discontinuities in family members' relationships with Alzheimer's patients. Family Relations. 1994;43:3–9. [Google Scholar]
- Cochrane JJ, Goering PN, Rogers JM. The mental health of informal caregivers in Ontario: an epidemiological survey. American Journal of Public Health. 1997;87:2002–2007. doi: 10.2105/ajph.87.12.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PG, Philip I, Mulle MA. Does the use of the geriatric depression scale make redundant the need for separate measures of well-being on geriatric wards? Age and Ageing. 1995;24:416–420. doi: 10.1093/ageing/24.5.416. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Winkens I, et al. Behavioural disturbances in dementia patients and quality of the marital relationship. International Journal of Geriatric Psychiatry. 2003;18:149–154. doi: 10.1002/gps.807. [DOI] [PubMed] [Google Scholar]
- Diener E. Subjective well-being. Psychological Bulletin. 1984;95:542–575. [PubMed] [Google Scholar]
- Dilworth-Anderson P, Gibson BE. The cultural influence of values, norms, meanings, and perceptions in understanding dementia in ethnic minorities. Alzheimer Disease & Associated Disorders. 2002;16:S56–S63. doi: 10.1097/00002093-200200002-00005. [DOI] [PubMed] [Google Scholar]
- Dwyer JW, Lee GR, Jankowski TB. Reciprocity, elder satisfaction, and caregiver stress and burden: The exchange of aid in the family caregiving relationship. Journal of Marriage and Family. 1994;56:35–43. [Google Scholar]
- Gallagher D, Nies G, Thompson LW. Reliability of the Beck Depression Inventory with older adults. Journal of Consulting and Clinical Psychology. 1982;50(1):152–153. doi: 10.1037//0022-006x.50.1.152. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Dal Canto PG, Jacob T, Thompson LW. A comparison of marital interaction patterns between couples in which the husband does or does not have Alzheimer’s disease. Journal of Gerontology: Social Sciences. 2001;56B:140–150. doi: 10.1093/geronb/56.3.s140. [DOI] [PubMed] [Google Scholar]
- Hellström I, Nolan M, Lundh U. Sustaining `couplehood': Spouses' strategies for living positively with dementia. Dementia. 2007;6:383–409. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hunsley J, Mash EJ. A guide to assessments that work. New York: Oxford University Press; 2008. [Google Scholar]
- Kramer BJ. Marital history and the prior relationship as predictors of positive and negative outcomes among wife caregivers. Family Relations. 1993;42:367–375. [Google Scholar]
- Lawrence RH, Tennstedt SL, Assmann SF. Quality of the caregiver–care recipient relationship: Does it offset negative consequences of caregiving for family caregivers? Psychology and Aging. 1998;13:150–158. doi: 10.1037//0882-7974.13.1.150. [DOI] [PubMed] [Google Scholar]
- Lee A, Browne M, Villanueva E. Consequences of using SF-12 and RAND-12 when examining levels of well-being and psychological distress. Australian & New Zealand Journal of Psychiatry. 2008;42(4):315–323. doi: 10.1080/00048670701881579. [DOI] [PubMed] [Google Scholar]
- LoboPrabhu SM, Molinari V, Lomax JW. Supporting the caregiver in dementia: a guide for health care professionals. Baltimore, MD: Johns Hopkins University Press; 2006. [Google Scholar]
- Lund DA, Pett MA, Caserta DS. Institutionalizing dementia victims: Some caregiver considerations. Journal of Gerontological Social Work. 1987;11:119–135. [Google Scholar]
- Lyons KS, Sayer AG, Archbold PG, Hornbrook MC, Stewart BJ. The enduring and contextual effects of physical health and depression on care-dyad mutuality. Research in Nursing & Health. 2007;30:84–98. doi: 10.1002/nur.20165. [DOI] [PubMed] [Google Scholar]
- McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. New York, NY: Oxford University Press; 1987. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris LW, Morris RG, Britton PG. The relationship between marital intimacy, perceived strain and depression in spouse caregivers of dementia sufferers. British Journal of Medical Psychology. 1988;61:231–236. doi: 10.1111/j.2044-8341.1988.tb02784.x. [DOI] [PubMed] [Google Scholar]
- Moss BF, Schwebel AI. Marriage and romantic relationships: Defining intimacy in romantic relationships. Family Relations. 1993;42:31–37. [Google Scholar]
- Netto NR, Jenny GYN, Philip YLK. Growing and gaining through caring for a loved one with dementia. Dementia. 2009;8:245–261. [Google Scholar]
- Noelker LS. Promoting positive relationships between nursing assistants and the families of cognitively impaired nursing home residents. Final report to the Cleveland Foundation. Cleveland, OH: A Benjamin Rose Institute; 1996. [Google Scholar]
- Norton MC, Piercy KW, Rabins PV, Green RC, Breitner JCS, Østbye T, et al. Caregiver–recipient closeness and symptom progression in Alzheimer Disease. The Cache County Dementia Progression Study. Journal of Gerontology B: Psychological and Social Sciences. 2009;64B(5):560–568. doi: 10.1093/geronb/gbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the Stress Process: An overview of concepts and their measures. The Gerontologist. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Perren S, Schmid R, Herrmann S, Wettstein A. The impact of attachment on dementia-related problem behavior and spousal caregivers' well-being. Attachment & Human Development. 2007;9:163–178. doi: 10.1080/14616730701349630. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology & Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Quinn C, Clare L, Woods B. The impact of the quality of relationship on the experiences and wellbeing of caregivers of people with dementia: A systematic review. Aging & Mental Health. 2009;13:143–154. doi: 10.1080/13607860802459799. [DOI] [PubMed] [Google Scholar]
- Schiaffino KM. Other measures of psychological well-being: The Affect Balance Scale (ABS), General Health Questionnaire (GHQ-12), Life Satisfaction Index-A (LSI-A), Rosenberg Self-Esteem Scale, Satisfaction with Life Scale (SWLS), and State-Trait Anxiety Index (STAI) Arthritis & Rheumatism. 2003;49:S165–S174. [Google Scholar]
- Schimmack U. The structure of subjective well-being. In: Eid M, Larsen RJ, editors. The science of subjective well-being. New York, NY: Guilford Press; 2008. pp. 97–123. [Google Scholar]
- Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. Journal of Clinical Psychology. 2009;65:467–487. doi: 10.1002/jclp.20593. [DOI] [PubMed] [Google Scholar]
- Spaid WM, Barush AS. Emotional closeness and caregiver burden in marital relationships. Journal of Gerontological Social Work. 1994;21(3–4):197–211. [Google Scholar]
- Spruytte N, Van Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychology and Psychotherapy: Theory, Research and Practice. 2002;75:295–311. doi: 10.1348/147608302320365208. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran C, Schwartz S, Treiber K, Norton MN, Green RC, et al. Progression in cognition, function and neuropsychiatric symptoms in a population cohort with Alzheimer’s dementia. The Cache County Dementia Progression Study. American Journal of Geriatric Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower R, Kasl S, Moritz D. The influence of spouse cognitive impairment on respondents' depressive symptoms: The moderating role of marital closeness. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1997;52B(5):S270–S278. doi: 10.1093/geronb/52b.5.s270. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Kiecolt-Glaser JK, Cacioppo JT. Construals of preillness relationship quality predict cardiovascular response in family caregivers of Alzheimer’s disease victims. Psychology and Aging. 1994;9:113–120. doi: 10.1037//0882-7974.9.1.113. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Walker AJ, Shin H, Bird DN. Perceptions of relationship change and caregiver satisfaction. Family Relations. 1990;39(2):147–152. [Google Scholar]
- Whitlatch CJ, Schur D, Noelker LS, Ejaz FK, Looman WJ. The stress process of family caregiving in institutional settings. The Gerontologist. 2001;41:462–473. doi: 10.1093/geront/41.4.462. [DOI] [PubMed] [Google Scholar]
- Williamson GM, Shultz R. Relationship orientation, quality of prior relationship, and distress among caregivers of Alzheimer's patients. Psychology and Aging. 1990;5(4):502–509. doi: 10.1037//0882-7974.5.4.502. [DOI] [PubMed] [Google Scholar]
- Wuest J, Ericson P, Stern P. Becoming strangers: the changing family caregiving relationship in Alzheimer's disease. Journal of Advanced Nursing. 1994;20(3):437–443. doi: 10.1111/j.1365-2648.1994.tb02378.x. [DOI] [PubMed] [Google Scholar]