Abstract
Resistance and relapse are still primary causes that result in poor effectiveness of chemotherapy in malignant gliomas. Therefore, development of new therapeutic strategies requires the identification of key molecular pathways regulating chemoresistance. We previously found that abnormal high expression of the Tie2 receptor in gliomas was associated with tumor malignancy. Here, we studied the role of Tie2 activation in drug resistance by testing the cytotoxicity of several chemotherapeutic drugs in a panel of human glioma cell lines and brain tumor stem cells and found that Tie2 activation was significantly related to chemoresistance. The essential role of Tie2 in this phenotype was illustrated by silencing Tie2 using specific siRNA, and the subsequent abrogation of the angiopoietin 1 (Ang1)-mediated chemoresistance. Using quantitative real-time PCR and functional drug efflux studies, we observed that Tie2 activation resulted in increased expression of ATP-binding cassette (ABC) transporters. Consistent with these results, downmodulation of ABCG2 or ABCC2 resulted in the inability of Tie2 activation to induce a chemoresistant phenotype. Our results indicate that Tie2 activation may be important in modifying the evolution of gliomas during conventional chemotherapy regimens, and open new avenues for the search of more effective therapies to avoid the inevitable brain tumor recurrence.
Keywords: Tie2, gliomas, chemoresistance, ABC transporters, brain tumor stem cells
Surgery and radiotherapy are not sufficient to induce long-lasting tumor regression in patients with malignant gliomas. Chemotherapy is therefore required for the successful treatment of these tumors. However, patients with malignant gliomas invariably experience a recurrence after therapy and exhibit a multidrug-resistant phenotype, with median survival times ranging from 9 to 12 months. This phenotype is defined as the simultaneous resistance of glioma cells to a broad spectrum of structurally unrelated cytotoxic drugs with different modes of action (Bredel, 2001; Dean et al., 2005). Overexpression of the ATP-binding cassette (ABC) transporters is thought to be central to the acquisition of multidrug-resistant phenotype (Bredel, 2001; Dean et al., 2005; Lu and Shervington, 2008). Understanding the regulation of ABC transporters in gliomas could lead to the development of strategies to target the multidrug-resistant phenotype. However, the signaling governing expression and function of ABC transporters in brain tumors remains elusive. Candidate molecules participating in the control of ABC transporters include tyrosine kinase receptors. Emerging evidence place Tie2 tyrosine kinase receptor in the extravascular compartment of several tumors including leukemia, gastric and thyroid tumors, and inflammatory breast cancer (Martin et al., 2008). Tie2 natural ligand, angiopoietin 1 (Ang1), is expressed in glioma cells and in mesenchymal and smooth muscle cells surrounding most blood vessels, and once secreted Ang1 binds extracellular matrix, an important component of tumoral microenvironment, establishing Ang1/Tie2 networks (Martin et al., 2008). Tie2 is a good candidate for the modulation of the tumor chemoresistance because its activation by Ang1 renders hematopoietic stem cells resistant to myelosuppressive stress, and Tie2+ cells in the niche were ultimately responsible for repopulating the bone marrow (Arai et al., 2004). Our group was the first to report aberrant Tie2 expression in neoplastic glial cells in more than 50% of malignant brain tumors and showed the existence of a Tie2-mediated functional signaling in these tumors (Lee et al., 2006, 2008).
In this report, we sought to determine if Tie2 signaling is related to the multidrug resistance of human glioma cells by upregulating the expression of several ABC transporters. To this end, we critically extended our studies to ascertain whether Tie2 is expressed in brain tumor stem cells (BTSCs). Analyses of Tie2 expression in a panel of BTSC (Jiang et al., 2007), generated by acute cell dissociation of glioblastoma surgical human samples (Galli et al., 2004; Singh et al., 2004; Jiang et al., 2007), showed Tie2 expression in the primary and secondary neurospheroid cultures, and in five of six BTSCs maintained in culture for more than 10 passages (Supplementary Figure 1). Expression of Tie2 in BTSCs may be of relevance because this population of cells is thought to be responsible for the recurrence of gliomas after treatment.
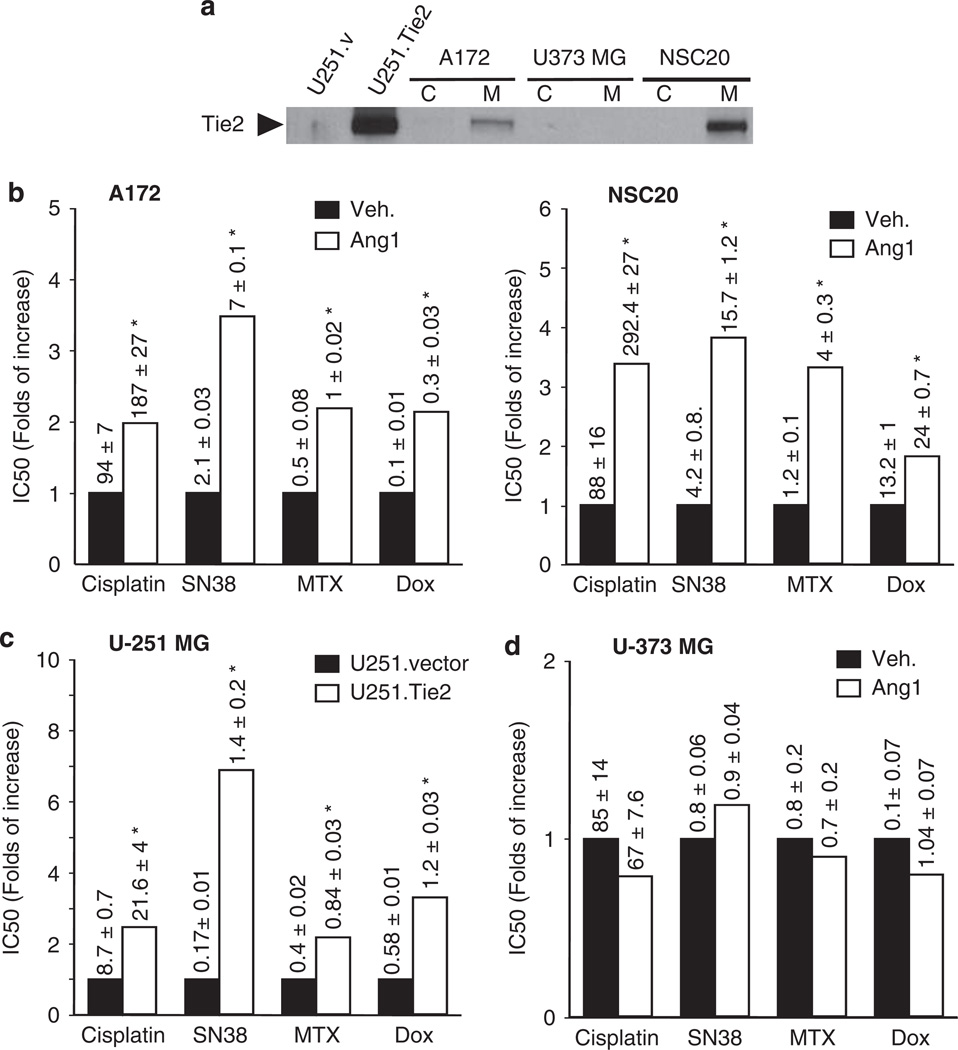
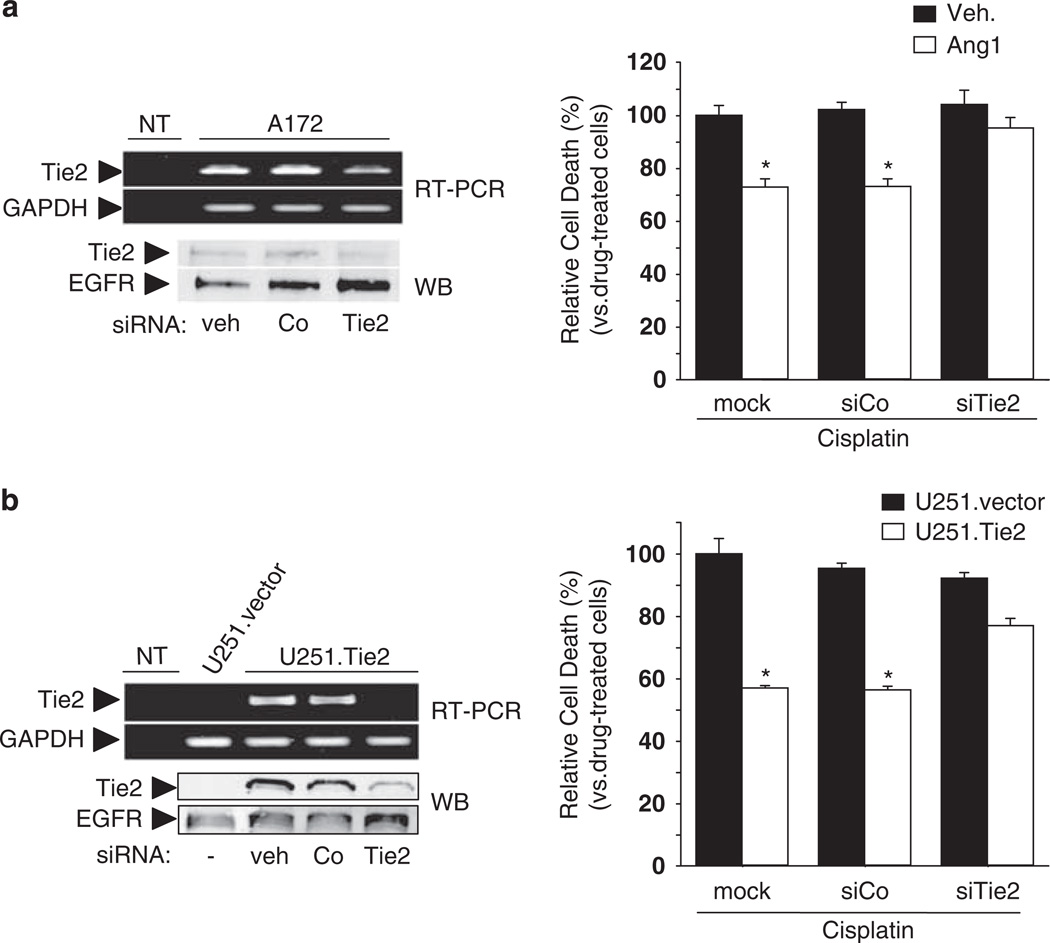
To study the effect of Tie2 in malignant glioma chemoresistance we selected a panel of cultures expressing endogenous Tie2 (A172 glioma cells and NSC20 BTSCs), Tie2− cells (U373 MG and U251 MG cells) and cells expressing constitutively active Tie2 (U251.Tie2; Figure 1a). Then, we treated the cultures with several chemotherapeutics with distinct mechanisms of action and calculated the IC50 doses for each of them. To activate Tie2, we added Ang1 to the A172 and NSC20 cells before treatment with cisplatin, SN38, doxorubicin and mitoxantrone. We showed that treatment with any of the drugs in cells expressing endogenous Tie2 resulted in up to fourfold increase in the IC50 upon Ang1 exposure (Figure 1b). Of interest, sorted Tie2+ BTSCs were more chemoresistant than sorted Tie2− BTSCs (Supplementary Figure 1c). We then tested the function of Tie2 in chemoresistance by comparing the effect of these drugs in the isogenic U251 MG cells. The IC50 values of the tested chemotherapeutic agents were up to sevenfold higher in the U251.Tie2 cells than in the U251.vector cells (Figure 1c). As expected, Ang1 did not significantly modify the IC50 values of any of the drugs in the Tie2− U373 MG cells (Figure 1d). To further ascertain the function of Tie2 in the chemoresistance phenotype, we downmodulated Tie2 RNA and protein levels using a Tie2-targeted siRNA previously validated by our group (Lee et al., 2006), and after Ang1 exposure, we treated the cultures with cisplatin. We found that the downregulation of Tie2 expression rendered A172 cells unable to acquire the Ang1-mediated chemoresistance (Figure 2a). Similar results were seen when Tie2 expression was knocked down in U251.Tie2 cells (Figure 2b). These data indicate that Tie2 activation is involved in the multidrug-resistant phenotype of malignant gliomas.
Figure 1.
Tie2 activation resulted in an increased IC50 of the different chemotherapeutic drugs in the human glioma cells. (a) Tie2 expression in glioma cells (A172, U373 MG) and brain tumor stem cells (NSC20). Cellular membrane fractions (M) were obtained as previously described (Lee et al., 2006), and subjected to western blotting analysis, using antibody against Tie2 (1:500, C-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Tie2 expression in isogenic cells (Lee et al., 2006), U251.vector and U251.Tie2 cells, was used as controls (C = cytosol fraction). (b) IC50 for the panel of chemotherapeutics in cells expressing endogenous Tie2. Cells (5000 cells per well in 96-well plates) were treated with angiopoietin 1 (Ang1, 250 ng/ml; R&D Systems, Minneapolis, MN, USA) or vehicle (0.1% bovine serum albumin, BSA) overnight, and then treated with the different drugs for 48 h (doses of 1–100 µm for cisplatin and 10 nm–5 µm for SN38, mitoxantrone (MTX), or doxorubicin (Dox)). Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and then we analysed the dose–response curves using CalcuSyn software version 2.1 (Biosoft, Cambridge, UK) and calculated the IC50 values (dose causing 50% cell survival) for each drug and treatment, as previously described (Alonso et al., 2007). Data are represented as the fold of increase in the IC50 relative to control (which was set equal to 1). Figure numbers represent mean ± s.d. from three independent experiments performed in triplicate. (c) U251.vector or U251.Tie2 cells overexpressing constitutively active Tie2 were treated with the indicated drugs for 48 h. Cell viability was assessed by MTT assay, and the IC50 values were calculated and represented as in Figure 1b. (d) Tie2− U373 MG cells were exposed to Ang1 for 18 h and then treated with the indicated panel of chemotherapeutics. Cell viability was assessed by MTT assay 48 h later, and the IC50 values were calculated and represented as in Figure 1b. *P < 0.01, compared to mock-treated cells (A172, NSC20, in b) or to U251.vector cells (in c) (t-test).
Figure 2.
Role of Tie2 in chemoresistance of glioma cells. (a) Left, reverse transcriptase (RT)–PCR and western blotting analyses showed downregulation of Tie2 mRNA and protein levels in A172 cells 48 h after transfection with Tie2 siRNA (40 nm; Santa Cruz Biotechnology), as compared to the controls (vehicle or control (Co) siRNA, 40 nm; Santa Cruz Biotechnology). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and epidermal growth factor receptor (EGFR) levels are shown as loading controls (NT = no DNA template). Methodology was reported previously (Lee et al., 2006). Right, A172 human glioma cells were transfected with siRNA against Tie2 or control siRNA or were mock treated. After 48 h, cultures were incubated overnight with angiopoietin 1 (Ang1, 250 ng/ml) or vehicle (veh) and then exposed to cisplatin (100 µm). After 2 days, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. Cell death is expressed relative to the cisplatin-induced cell death in untreated A172 cells (= 100%). Data are represented as the mean ± s.d. from three independent experiments performed in triplicate. (b) Left, RT–PCR and western blotting analyses showed downregulation of Tie2 mRNA and protein in U251.Tie2 cells 48 h after transfection with Tie2 siRNA (40 nm), as compared to the controls (veh or control (Co) siRNA). Right, U251.Tie2 and U251.vector glioma cells were transfected with siRNA against Tie2 or control siRNA or were mock treated. After 48 h, cultures were exposed to cisplatin (25 µm). After 2 days, cell viability was measured by MTT reduction assay. Cell death is expressed relative to cisplatin-induced cell death in untreated U251.vector cultures (= 100%). Data are represented as median ± s.d. from three independent experiments performed in triplicate. *P < 0.01 in either vehicle-treated A172 cells (as in a) or U251.vector cells (as in b) (one-way analysis of variance (ANOVA) followed by a Student—Newman–Keuls multiple range test).
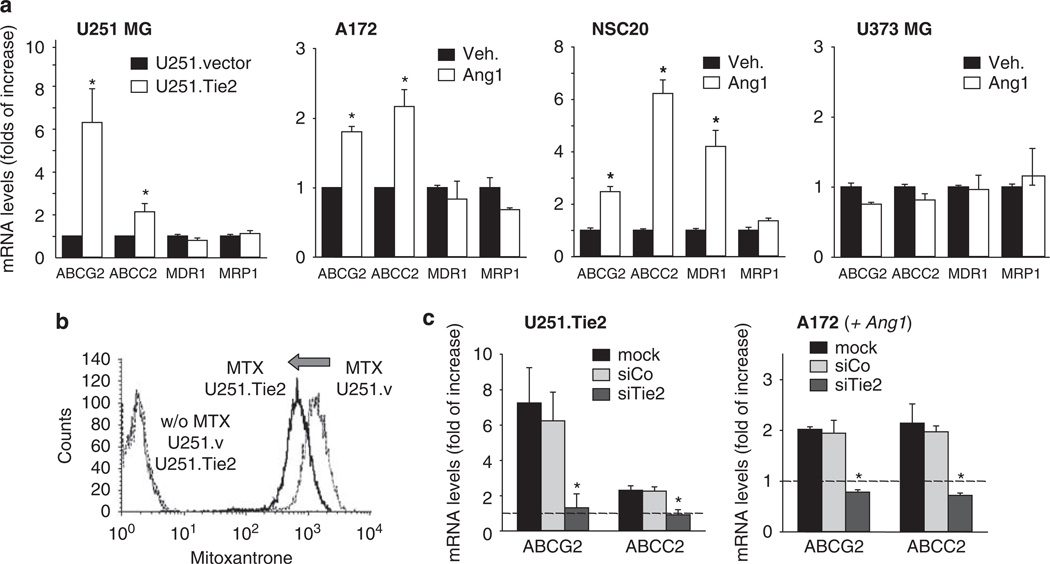
One of the main mechanisms responsible for multi-drug resistance in cancer cells is related to the aberrant expression of ABC transporters (Gottesman et al., 2002). We next sought to analyse whether Tie2 activation modifies the expression levels of ABC transporters. To this end, we first compared the mRNA levels of ABC transporters in the isogenic cell lines U251.vector and U251.Tie2 cells. We observed that the expression levels of ABCG2/Bcrp1/MXR and ABCC2/MRP2 transcripts were significantly higher in U251.Tie2 cells than in U251.vector cells. No significant differences were observed in the RNA levels of ABCB1/MDR1 or ABCC1/MRP1 between both cell lines, suggesting specificity (Figure 3a). A second set of experiments was performed in cells with endogenous Tie2 (A172 and NSC20) and Tie2− cells (U373 MG). In these experiments, we observed that Ang1 positively modulated the expression levels of ABCG2 and ABCC2 in A172 cells and ABCG2, ABCC2 and MDR1 in the BTSC NSC20 (Figure 3a). As expected, Ang1 treatment of the Tie2− U373 MG cells did not result in significant modifications in the expression levels of any of the ABC transporters tested (Figure 3a). These results were confirmed by analysing protein levels of ABCG2 and ABCC2 in A172 (Tie2+) and U373 MG (Tie2−) cells (Supplementary Figure 2a). We were next interested in ascertaining whether Tie2 activation was associated with a drug efflux increase. We found that Tie2 activation resulted in a decrease in the intracellular content of the autofluorescent drug, mitoxantrone. Specifically, U251.Tie2 cells showed a significant decrease in drug accumulation compared to U251.vector cells (100 ± 2.9 vs 64.7 ± 2%; Figure 3b) after the drug efflux phase. Similar results were observed in A172 cells following Ang1 treatment (100 ± 2.22 vs 80 ± 1.71% of vehicle-treated A172 cells; Supplementary Figure 2b), revealing an enhanced functionality of the ABC transporters. To further assess the function of Tie2 in the regulation of ABCG2 and ABCC2, we downregulated Tie2 expression using specific siRNA, and analysed the RNA of both transporters using qPCR. Downmodulation of Tie2 expression by specific siRNA completely blocked the Tie2-mediated increase of ABCG2 and ABCC2 in A172 and U251.Tie2 cell lines (Figure 3c). These results showed that the Ang1/Tie2 axis modulates the expression and function of ABC transporters in glioma cells and BTSCs. Finally, we asked whether other ligands of tyrosine kinase receptors different from Tie2 and commonly overexpressed in gliomas, such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), regulate the expression of ABC transporters. To this end, we treated Tie2-expressing A172 cells with EGF and bFGF and analysed the ABCG2 and ABCC2 RNA levels by qPCR. These experiments revealed that Ang1 but no EGF or bFGF increased ABC transcript levels, strongly suggesting that the Tie2 pathway has specific regulatory function in the expression of the ABC transporters (Supplementary Figure 3).
Figure 3.
Tie2 activation resulted in upregulation of ATP-binding cassette (ABC) transporters expression levels in human glioma cells and brain tumor stem cells. (a) ABC transporter RNA expression (ABCG2, ABCC2, MDR1, MRP1) in human glioma cells as assessed by qPCR. Glioma cells (A172 and U373 MG cells) and brain tumor stem cells (NSC20) were treated with either angiopoietin 1 (Ang1) or vehicle. Total RNA was extracted 18 h later and qPCR was performed. Relative expression levels of ABCG2, ABCC2, MDR1 and MRP1 are represented as the fold increase compared to basal levels (= 1). The mean ± s.d. are shown from three independent experiments performed in triplicate. *P < 0.01 Ang1- vs vehicle-treated cells (A172 and NS20) and U251.Tie2 vs U251.vector cells (t-test). (b) Active Tie2 is related to increased drug efflux. U251.vector (Tie2−) and U251.Tie2 (Tie2+) cells were treated with mitoxantrone (MTX, 1 µm) or vehicle for 30 min, at 37 °C in a 5% CO2 atmosphere. Cells were then incubated for 1 h at 37 °C in mitoxantrone-free medium, and then analysed with a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). Shown are representative results from one of the three independent experiments. (c) Examination of ABCG2 and ABCC2 RNA levels of glioma cells after Tie2 downmodulation. Cells were transfected with siRNA against Tie2 (siTie2; 40 nm) or control siRNA (siCo; 40 nm) or were mock treated. After 48 h, A172 cells were incubated overnight with Ang1 (250 ng/ml) or vehicle. Total RNA was extracted, and the ABCG2 and ABCC2 RNA levels were measured by qPCR. Relative expression levels of ABCG2 and ABCC2 are represented as the x-fold increase compared to the basal expression levels in U251.vector or vehicle-treated A172 cells (= 1, dotted lines). Shown are the mean ± s.d. from three independent experiments performed in triplicate. *P < 0.01 vs mock-treated cells (one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls multiple range test).
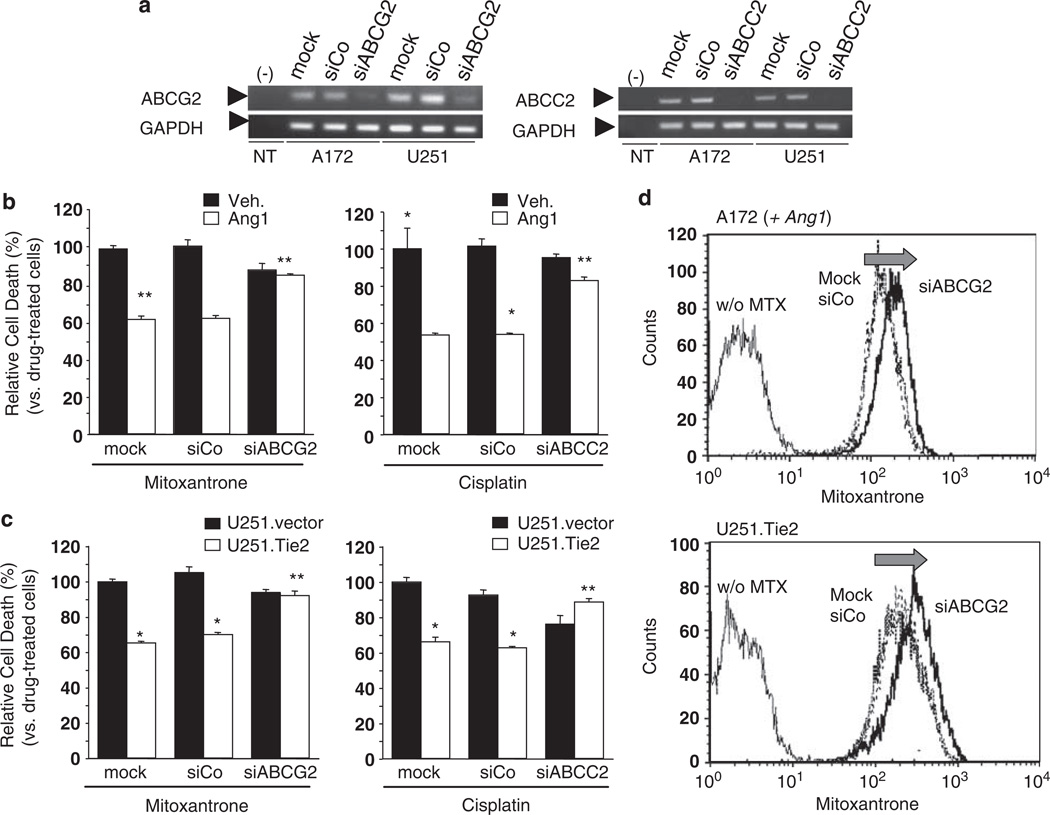
To dissect the specific function of ABCG2 and ABCC2 in the Tie2-mediated chemoresistant phenotype of malignant gliomas, we downregulated ABCG2 and ABCC2 expression using specific siRNAs (Figure 4a; Supplementary Figure 4). We then tested the cytotoxicity of two drugs whose membrane transport has been described as dependent of ABCG2 (mitoxantrone) or ABCC2 (cisplatin). We observed that downmodulation of ABCG2 expression completely suppressed Tie2-mediated protection of mitoxantrone-induced cell death. Importantly, decrease in the Tie2-mediated resistance to cisplatin was also observed after ABCC2 downmodulation (Figures 4b and c). To further analyse the effect on the levels of ABCG2 in Tie2-mediated chemoresistance, we performed a mitoxantrone efflux assay in Tie2-expressing cells after downmodulation of ABCG2 expression. ABCG2 silencing resulted in a marked increase of the intracellular accumulation of drug (36.70 ± 2.16% increase in the A172 cells and 26.40 ± 1.02% in U251.Tie2 cells) (Figure 4d). Collectively, these data indicate that Tie2 mediates the chemoresistant phenotype of malignant glioma cells by increasing the expression and function of the ABC transporters.
Figure 4.
Tie2-induced chemoresistance is mediated by upregulation of ATP-binding cassette (ABC) transporters. (a) Reverse transcriptase (RT)–PCR showing a decrease in the ABCG2 and ABCC2 RNA levels in the A172 and U251 MG cells 48 h after transfection of siRNA against ABCG2 (siABCG2), siRNA against ABCC2 (siABCC2), control siRNA (siCo) (50 nm; Ambion Inc., Applied Biosystems, Foster City, CA, USA), or mock-treated cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification was used as the internal control. NT, no DNA template. (b) Drug-induced cell death in A172 human glioma cells after incubation with ABCG2 siRNA or ABCC2 siRNA. Cells were then treated overnight with angiopoietin 1 (Ang1, 250 ng/ml) or vehicle, and mitoxantrone (500 nm) or cisplatin (100 µm) were added to the cultures. After 48 h, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. The relative drug-induced cell death is represented as the mean ± s.d. of three independent experiments performed in triplicate (cell death induced by drug in untreated A172 was set equal to 100%). (c) Drug-induced cell death in the U251 MG cells treated with mitoxantrone (500 nm) after incubation with ABCG2 siRNA, or cisplatin (10 µm) after incubation with ABCC2 siRNA. After 48 h, cell viability was measured by MTT reduction assay. Relative drug-induced cell death is represented as the mean ± s.d. of three independent experiments performed in triplicate (cell death induced by drug in mock-treated U251.vector was set equal to 100%). *P < 0.01 vs either vehicle- or mock-treated (A172) or U251.vector cells (one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls multiple range test). (d) Mitoxantrone incorporation functional study in Ang1-stimulated A172 and U251.Tie2 cells after downmodulating ABCG2 expression. Cells were transfected with siRNA against ABCG2 or control siRNA (both 50 nm), and 48 h later, they were treated with mitoxantrone (MTX; 1 µm) or the vehicle for 30 min. Cells were then washed, allowed to incubate for 1 h in mitoxantrone-free media and then fluorescence was determined as explained in Figure 3. Shown are representative results from one of the three independently performed experiments.
In the present study, we found that the tyrosine kinase receptor Tie2 modulated human glioma cell and BTSC resistance to cell death induced by several structurally unrelated drugs. We also found that the Ang1/Tie2-mediated chemoresistant phenotype was related to the upregulation of the expression levels of several ABC transporters that have been shown to be overexpressed in gliomas and stem cells (Decleves et al., 2006; Jin et al., 2008; Lu and Shervington, 2008; Shervington and Lu, 2008). These data may be relevant to explain the mechanism of chemoresistance in gliomas. Because BTSCs are most probably the cause of tumor recurrence after treatment (Dean et al., 2005), further studies are required to determine whether the expression of Tie2 in this population of cells is one of the factors propelling recurrence after chemotherapy. Noteworthy, hematopoietic cells expressing Tie2 are responsible for the repopulation of the bone marrow after genotoxic stress (Arai et al., 2004).
The expression of ABC transporters is markedly upregulated in both the blood vessels and parenchyma of malignant gliomas (Zhang et al., 2003). Intriguingly, Tie2 expression is also found in both tumor endothelial cells and neoplastic glial cells (Zadeh et al., 2004; Lee et al., 2006). Coincidence of an aberrant expression of Tie2 and ABC transporters in both compartments suggests a potential functional relationship. Of practical relevance, pharmacological targeting of Tie2 activity could modulate the drug efflux in endothelial and glioma parenchyma increasing drug intratumoral concentration and the efficacy of chemotherapy. In addition, we believe that because Tie2 has been reported to be expressed in the nonstromal compartment of several cancers, including leukemia, gastric tumors, thyroid tumors and inflammatory breast cancer (Martin et al., 2008), our results might have repercussions not only in the brain tumor field, but also in envisioning the therapy for other types of tumors.
Supplementary Material
Acknowledgements
We thank Alyson Todd (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center) for editorial assistance. This work was supported by a postdoctoral fellowship from the Department of Education and Science, Spain (to VM), an American Brain Tumor Association postdoctoral fellowship (to DL), Elsa U Pardee Foundation Award (to CGM), and an NIH/Developmental Research Program P50CA127001 (to CGM).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Bredel M. Anticancer drug resistance in primary human brain tumors. Brain Res Brain Res Rev. 2001;35:161–204. doi: 10.1016/s0165-0173(01)00045-5. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Decleves X, Amiel A, Delattre JY, Scherrmann JM. Role of ABC transporters in the chemoresistance of human gliomas. Curr Cancer Drug Targets. 2006;6:433–445. doi: 10.2174/156800906777723930. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- Jin F, Zhao L, Zhao HY, Guo SG, Feng J, Jiang XB, et al. Comparison between cells and cancer stem-like cells isolated from glioblastoma and astrocytoma on expression of anti-apoptotic and multidrug resistance-associated protein genes. Neuroscience. 2008;154:541–550. doi: 10.1016/j.neuroscience.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Lee OH, Xu J, Fueyo J, Alonso MM, Liu D, Martin V, et al. Angiopoietin-2 decreases vascular endothelial growth factor expression by modulating HIF-1 alpha levels in gliomas. Oncogene. 2008;27:1310–1314. doi: 10.1038/sj.onc.1210731. [DOI] [PubMed] [Google Scholar]
- Lee OH, Xu J, Fueyo J, Fuller GN, Aldape KD, Alonso MM, et al. Expression of the receptor tyrosine kinase Tie2 in neoplastic glial cells is associated withintegrin beta1-dependent adhesion to the extracellular matrix. Mol Cancer Res. 2006;4:915–926. doi: 10.1158/1541-7786.MCR-06-0184. [DOI] [PubMed] [Google Scholar]
- Lu C, Shervington A. Chemoresistance in gliomas. Mol Cell Biochem. 2008;312:71–80. doi: 10.1007/s11010-008-9722-8. [DOI] [PubMed] [Google Scholar]
- Martin V, Liu D, Fueyo J, Gomez-Manzano C. Tie2: a journey from normal angiogenesis to cancer and beyond. Histol Histopathol. 2008;23:773–780. doi: 10.14670/HH-23.773. [DOI] [PubMed] [Google Scholar]
- Shervington A, Lu C. Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Invest. 2008;26:535–542. doi: 10.1080/07357900801904140. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Zadeh G, Qian B, Okhowat A, Sabha N, Kontos CD, Guha A. Targeting the Tie2/Tek receptor in astrocytomas. Am J Pathol. 2004;164:467–476. doi: 10.1016/S0002-9440(10)63137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–2087. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.