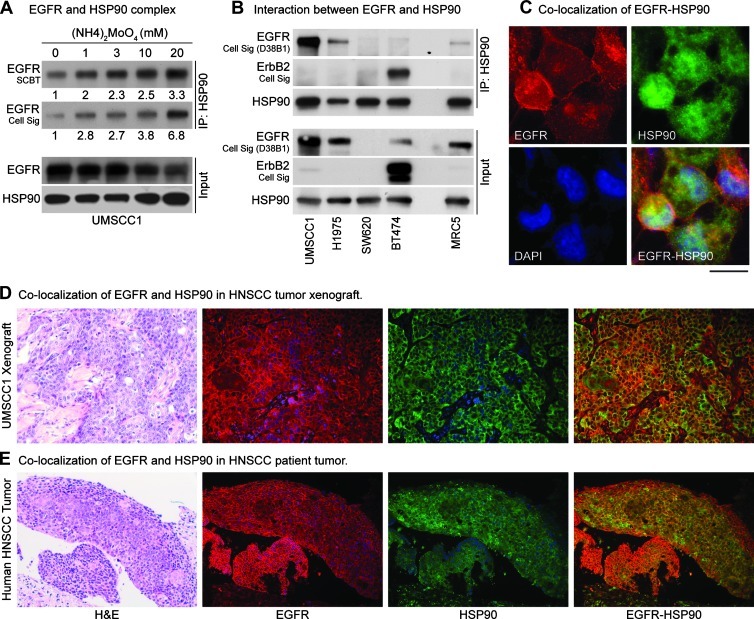
Figure 1.
Interaction of WT-EGFR with HSP90. Immunoprecipitation using anti-HSP90 antibody revealed minimum EGFR in the complex; therefore, ammonium molybdate (20 mM), which is known to stabilize the interaction of HSP90 with its client proteins, was added to the lysis buffer. (A) IP results showed an increase in EGFR-HSP90 interaction with increasing concentrations of ammonium molybdate. Densitometric analysis of the films was performed using ImageJ software, and relative intensity is shown. Two EGFR antibodies were used to confirm these results. (B) Interaction between HSP90 and EGFR was assessed in five cell lines, representing normal EGFR-expressing cells (MRC5), EGFR-negative (SW620), ErbB2-driven, EGFR-independent (BT474), erlotinib-resistant (T790M-EGFR; NCIH1975), and EGFR-amplified (UMSCC1) tumor cells. To address the issue of cross-reactivity, multiple EGFR antibodies were used, and their pattern was compared with ErbB2. One EGFR and one ErbB2 antibody were selected for the IP experiments. UMSCC1 and NCI-H1975 cell lines showed maximum interaction between EGFR with HSP90, whereas BT474 and MRC5 cells showed minimal interaction. EGFR-HSP90 interaction was absent in EGFR null SW620 cells. Colocalization of EGFR and HSP90 in tumor cells, xenografts, head and neck cancer patient's tumor was assessed by dual immunostaining for EGFR and HSP90. (C) UMSCC1 cells showed modest colocalization of these two proteins, which was also confirmed in (D) xenograft and (E) patient tumor specimen.