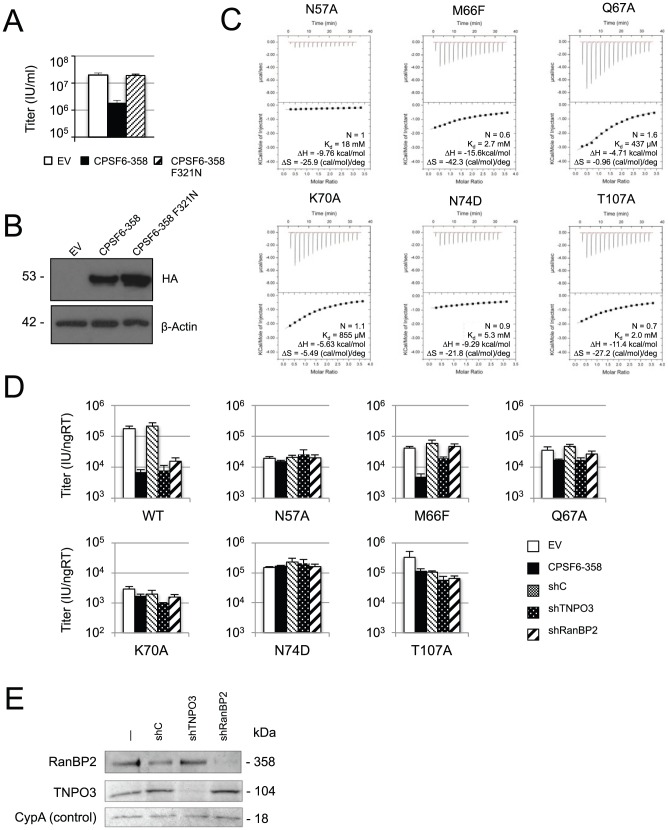
Figure 6. The CPSF6-binding interface determines HIV-1 nuclear entry requirements.
(A–B) CPSF6 residue F321 is critical for interaction with HIV-1 CAN. (A) HeLa cells expressing empty vector (white bar), HA-tagged CPSF6-358 (black bar) or CPSF6-358 bearing mutation F321N (striped bar) were infected with GFP-encoding VSV-G pseudotyped HIV-1 vector. F321N abolished restriction by CPSF6-358, confirming the importance of this residue in the HIV-1 CAN:CPSF6 interaction. (B) Western blot to show CPSF6-358 and CPSF6-358 F321N expression levels, with actin as loading control. (C) ITC of CPSF6313–327 against mutant HIV-1 CANs. All mutations at the CPSF6-binding interface resulted in reduced affinity to CPSF6313–327. The stoichiometry (N), affinity (Kd), enthalpy (ΔH) and entropy (ΔS) of interaction are shown. (D) Titres of VSV-G pseudotyped GFP-encoding HIV-1 vectors bearing wild type or mutant CA on HeLa cells expressing empty vector (EV), CPSF6-358, control knockdown cells (shC) and cells depleted for TNPO3 (shTNPO3) or RanBP2 (shRanBP2). The data are representative of two independent experiments, each using three different virus doses. Mutation of HIV-1 CAN residues involved in binding to CPSF6 resulted in the loss of dependence on TNPO3 and RanBP2, suggesting a link between CPSF6 binding and normal nuclear import of HIV-1. (E) Western blot to show knockdown of TNPO3 and RanBP2, with cyclophilin A (CypA) as loading control.