Abstract
Cyclin-dependent kinase 5 (Cdk5) plays a pivotal role in neuronal migration and differentiation, and in axonal elongation. Although many studies have been conducted to analyze neuronal functions of Cdk5, its kinase activity has also been reported during oligodendrocyte differentiation, which suggests Cdk5 may play an important role in oligodendrocytes. Here, we describe a hypomyelination phenotype observed in Emx1-cre mediated Cdk5 conditional knockout (cKO) mice (Emx1-cKO), in which the Cdk5 gene was deleted in neurons, astrocytes and oligodendrocyte -lineage cells. In contrast, the Cdk5 gene in CaMKII cKO mice was deleted only in neurons. Because the development of mature oligodendrocytes from oligodendrocyte precursor cells is a complex process, we performed in situ hybridization using markers for the oligodendrocyte precursor cell and for the differentiated oligodendrocyte. Our results indicate that hypomyelination in Emx1-cKO is due to the impaired differentiation of oligodendrocytes, rather than to the proliferation or migration of their precursors. The present study confirmed the in vivo role of Cdk5 in oligodendrocyte differentiation.
Keywords: Cyclin-dependent kinase 5, Oligodendrocyte differentiation, Hypomyelination, Conditional knockout mice
Introduction
Cyclin-dependent kinase (Cdk) is a family of many kinases that regulate the cell cycle division. Cdk5 is an exceptional member of the Cdk5 family and plays a pivotal role in neuronal functions. Cdk5 is expressed in various cells and forms a heterodimer with one of its activators, either p35 or p39. p35 and p39 can be digested by a calcium activated protease, known as calpain, into p25 and p29, respectively [1]. p35 and p39 are predominantly expressed in the nervous system. The Cdk5/p35 complex plays an important role in brain development, neuronal migration and neuronal differentiation, and axonal elongation [2–5]. Earlier studies showed that a lack of Cdk5 in conventional knockout mice led to neuron migration defects and prenatal lethality, and the similar phenotype was observed in p35; p39 double mutant mice [6, 7]. The Cdk5 mutant mice showed inverted cerebral cortical layers due to defective neuronal migration [6, 8]. Cdk5 is also known to be involved in axonal elongation; a high level of temporal correlation is observed in p35 expression, Cdk5 kinase activity and formation of axon tracts in the developing brain [9, 10]
Oligodendrocytes are the myelinating cells of the central nervous system. Matured oligodendrocytes are generated after undergoing a complex and precisely timed program of proliferation, migration, differentiation, and myelination to form an insulating sheath on the axons [11–16]. Due to the complex and highly orchestrated developmental process, oligodendrocytes are considered among the most vulnerable cells in central nervous system. Various internal and external factors can lead to oligodendrocyte and myelin loss, and can induce human demyelinating disorders such as Multiple Sclerosis. Although many studies focusing on the role of Cdk5 in neurons have been conducted, only a few have reported the function of Cdk5 in glial cells. It was reported that the differentiation of oligodendrocyte precursor cells (OPC) is accompanied by the increased level of Cdk5 kinase activity [17]. Another showed that Cdk5 regulates OPC migration and differentiation in vitro [18]. Because these studies were conducted in cultured cells in vitro, the question of whether Cdk5 plays a pivotal role in oligodendrocytes in vivo still needs to be explored.
We previously reported on the generation of Emx1-cre mediated Cdk5 conditional knockout (Emx1-cKO) mice, as well as on the generation of CaMKII-cre mediated cKO (CaMKII-cKO) [19]. In Emx1-cKO mice, we selectively deleted Cdk5 in the neuronal lineage of the neocortex and hippocampus, and in the astrocyte and oligodendrocyte lineage of the pallium [20, 23]. In CaMKII-cKO, Cdk5 was selectively deleted only in the neuronal lineage cells. Emx1-cKO mice showed growth retardation and died around P14; CaMKII-cKO mice also showed growth retardation and died around P21. Interestingly, we found hypomyelination in Emx1-cKO mice, but not in CaMKII-cKO mice, and hypothesized that the removal of Cdk5 from oligodendrocyte lineage would lead to defects in oligodendrocyte development. We further determined that the hypomyelination in Emx1-cKO is due to the impaired differentiation of oligodendrocytes, rather than to proliferation or migration of OPC.
Experimental Procedure
Cdk5 Mutant Mice
The mice used in the experiments were housed in accordance with protocols approved by the Institutional Animal Care and Use Committee at RIKEN Brain Science Institute and Waseda University. Cdk5 conventional KO mice (Cdk5−/−) were generated as described [5]. Mice carrying loxp-flanked Cdk5 allele were generated and maintained as described [21]. Emx1-cre mice were generated as described [22]. Cdk5loxp/loxp mice was bred with Emx1-cre mice to generate Cdk5loxp/+; Emx1cre/+ mice, which were then crossed with Cdk5loxp/loxp mice to produce Emx1-cKO offspring (Cdk5loxp/loxp; Emx1cre/+). CaMKII-cre transgenic mice used in this study carried a cre transgene under the transcriptional control of the CaMKIIα promoter. Cdk5loxp/loxp mice were crossed with the CaMKII-cre mice line to generate Cdk5loxp/+; CaMKII-cre mice, which were then bred with Cdk5loxp/loxp mice to produce CaMKII-cKO offspring (Cdk5loxp/loxp;CaMKII-cre). Cdk5loxp/loxp mice developed and behaved the same as wild type mice, and were used as control [19]. Genotypes of the mice were determined by PCR as described [19, 21, 22].
Bromodeoxyuridine Injection, 1′1-Dioctadecyl-3-3-3′-3′-Tetramethylindiocarbocyanine Perchlorate (DiI) Labeling and Immunostaining
For 5-bromo-2′-deoxyuridine (BrdU) injection, Emx1-cKO mice were injected with BrdU at postnatal day 9 (40 ug/g body weight, intraperitoneal) and analyzed at postnatal day 14. Mice were given inhalation anesthesia using diethyl ether and were then perfused transcardically with 4% PFA in PBS. Brain samples were fixed in 4% PFA in PBS overnight, after perfusion. After dehydration in 20% sucrose, the samples were embedded in O.C.T. compound. Cryosections were cut at 14 μm and stored at minus 20 degrees until being used for immunostaining.
For immunostaining, before applying mouse monoclonal anti-BrdU antibody (1:200, Dako), the sections were incubated with 4 N HCl to expose the BrdU labeled DNA. After incubating with anti-BrdU antibody at 4 degrees overnight, secondary antibody (AlexaFluor-488 goat anti-mouse IgG, 1:1000) was applied. After washing with PBS, the sections were mounted with Vectashield (Vector Labs, Burlingame, CA). SMI-31 (Sternberger Monoclonals Inc. MD, USA) which recognizes phosphorylated-neurofilament was used with a dilution of 1:1000. Secondary antibody was visualized by DAB reaction product as specified by Vectastain Elite protocol (Vector Laboratories, CA, USA). All the images were captured by a microscope (Olympus, DP70). Area was measured by Image J 1.43. Data were expressed as Mean ± S.E.M. and analyzed by Student’s t test. P < 0.05 was considered statistically significant.
DiI labeling was performed as described preciously [23]. After 3 weeks in fixative in dark at 37°C, the brains were embedded in 2% agarose, cut into 100 μm sections and photographed with a rhodamine filter.
In situ Hybridization
To obtain a cDNA fragment for oligodendrocyte/OPC related genes, the following primer sets were used for RT–PCR: MBP (forward, 5′-CACACACGAGAACTACCCA-3′; reverse, 5″-AACACTGTGGAGCTTGTGG-3′), MAG (forward, 5′-CTCTATGGCACCCAGAGCCT-3′; reverse, 5′-TGTCCTTGGTGGGTCGTTTT-3′), Olig2 (forward, 5′-TCAGAGCACAGGAGCAAGC-3′; reverse, 5′-AACGAC ACAGAAAGAAAACAGC-3′), PDGFRa (forward, ACCT TGCACAATAACGGGAG-3′; reverse, 5′-GAAGCCTTT CTCGTGGACAG-3′), CNP (forward, 5′-CCAAATTC TGTGACTACGGG-3′; reverse, GGTTTGCCCTTCCCAT AGTA-3′) and PLP (forward, 5′-GAAAAGCTAATTGA GACCTA-3′; reverse, 5′-GAGCAGGGAAACTAGTG TGG-3′). Amplified fragments were subcloned into pGEMT-easy vector (Promega, Madison, Wisconsin) and verified by DNA sequencing. Digoxigenin-labeled riboprobes were synthesized and in situ hybridization was performed as described [23].
Western Blot Analysis
The cerebral cortices and cerebellums of the mice were collected from Emx1-cKO mice and their littermates at postnatal day 14, and the same was done with CaMKII-cKO mice and their littermates. Western blot analysis was conducted as previously described [23]. Briefly, brain samples were homogenized with TNN buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40) containing 0.2 mM Na3VO4, 50 mM NaF, complete protease inhibitor cocktail (Roche Applied, Berkeley, CA) and phosphatase inhibitor cocktail. The protein concentration in each sample was determined using the BSA protein assay. Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacryl-amide gel electrophoresis (SDS–PAGE) gel, and the proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, USA). The membranes were blocked in 3% skim milk in TBS buffer containing 0.01% Tween20 (TBST), then incubated with monoclonal antibody against CNPase (1:1000, Chemicon International) at 4°C overnight. After washing with TBST, membranes were incubated with secondary antibodies of anti-mouse IgG conjugated to HRP (1:1000, Santa Cruz). After reaction with the enhanced chemiluminescence (ECL) plus reagent, a signal was detected by LAS-3000 (Fujifilm, Japan). The same membrane was re-blotted with a polyclonal antibody against actin (1:1000, Sigma) and followed by a secondary antibody of goat anti-rabbit IgG HRP conjugate (1:1000, Santa Cruz). ECL plus a detection kit was used to develop the membrane. The membrane was exposed and images were processed with a LAS 3000. Densitometry analysis was performed with Multi Gauge V2.3. Data were obtained from 3 independent experiments. Statistical analysis was conducted by the Student’s t test and Mean ± S.E.M. is shown in the graph. P < 0.05 was considered statistically significant.
Electron Micrograph
Images from electron microscopy were obtained as described [24]. Briefly, brains from the control and mutant mice at postnatal day 14 were transcardially perfused with 0.9% NaCl (200 ml/kg body weight), followed by 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (PB) for 15 min. They were then postfixed with 2% (w/v) osmium tetroxide in 0.1 M PB for 1 h, dehydrated in a graded ethanol series, and flat-embedded in epoxy resin (Araldyte CY212, TAAB, UK). Eighty nm-thick ultrathin sections (80 nm Ultracut-UCT, Leica, Austria) were stained with uranyl acetate and lead citrate. Electron micrographs were recorded on imaging plates through an LEO 912 electron microscope (LEO, Germany). Images were scanned and digitized using an FDL 5000 imaging system (Fuji Film, Japan).
Results
Decreased Level of Myelination-Related Protein from Cerebral Cortices of Emx1-cre Mediated Mice but not from CaMKII-cre Mediated Cdk5 Conditional Knockout Mice
We previously reported the generation of Emx1-cKO mice and described abnormal laminar structures in the cerebral cortex and hippocampus [23]. Emx1-cKO mice showed growth retardation and died around P14 [23]. We suspected that the myelin system had serious defects that led to postnatal death, and we analyzed the protein level of CNPase, which is one of the myelin-related proteins, using the cerebral cortices and cerebella homogenate from Emx1-cKO mice and the littermate controls. Significant reduction in CNPase protein was observed in cerebral cortices of Emx1-cKO when assayed by western blot, whereas the expression of CNPase in cerebella of Emx1-cKO was nearly the same as it was in littermate controls (Fig. 1a). Since Cdk5 in the Emx1-cKO mice was deleted not only in neurons but also in the astrocytes and oligodendrocytes of pallium, we analyzed the expression level of CNPase in cerebral cortices and cerebella from CaMKII-cKO [19] by assaying western blot, in order to further investigate whether the myelin defect resulted from lacking Cdk5 in oligodendrocytes or in neurons. Interestingly, we found that the CNPase expression level in both cerebral cortices and cerebella of CaMKII-cKO mice appeared similar to the littermate controls (Fig. 1b). Cumulatively, the data suggests that the myelin system is significantly affected in Emx1-cKO mice, mainly due to the deletion of Cdk5 in oligodendrocyte lineage rather than in neuronal lineage.
Fig. 1.
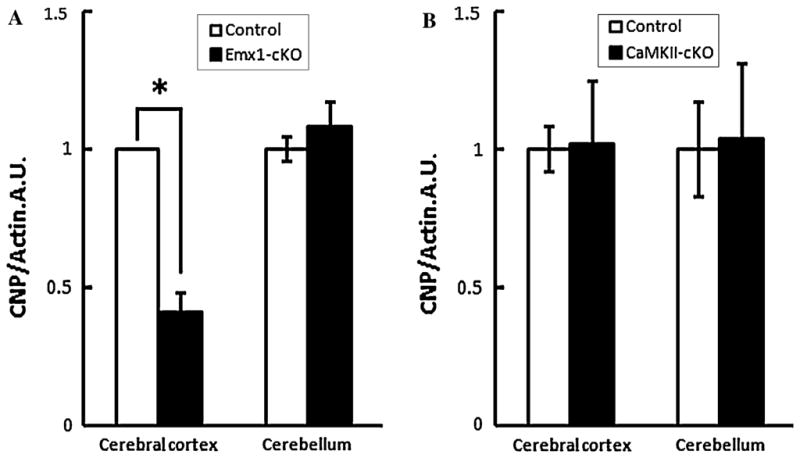
Decreased level of myelination-related protein from cerebral cortices of Emx1-cre mediated Cdk5 cKO (Emx1-cKO) mice, but not from CaMKII-cre mediated Cdk5 cKO (CamKII-cKO) mice. Western blotting analysis of CNPase expression. Protein levels for CNPase and actin were examined using cerebral cortices and cerebella from Emx1-cKO and control mice at postnatal day 14, CaMKII-cKO and control mice at postnatal day 15. Blots were incubated with anti-CNPase (1:1000) or anti-Actin (1:1000) antibodies. For Emx1-cKO mice (a), distinct reduction in CNPase/actin ratio of cerebral cortex was observed in western blotting compared with control mice (*P < 0.05), while the CNPase/actin ratio of cerebellum was not significantly altered compared with control mice. For CaMKII-cKO mice (b), the CNP/Actin ratio had no significant change in the cerebral cortex or the cerebellum compared with control mice
Olig2 and PDGFRα Expression Pattern in Cdk5−/− Mice Brain
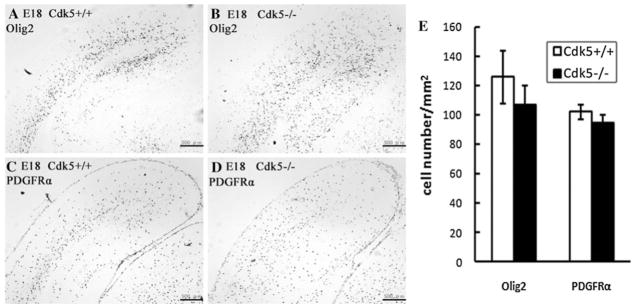
As the formation of the myelin sheath is a complicated process, there could be various reasons for the myelin defect in Emx1-cKO mice. Therefore, in order to investigate whether the reduction of myelin is a result of a failure to generate OPCs or a disturbance of the normal maturation program, we performed in situ hybridization with Cdk5−/− mice and the littermate controls Cdk5+/+ by using Olig2 and PDGFRα as markers for OPCs and undifferentiated oligodendrocytes, respectively. Oligodendrocytes are generated after the completion of neuron migration from the precursors located in the subventricular zone and cortical plate, and then these precursor cells migrate to the intermediate zone, proliferate again, and then differentiate to mature oligodendrocytes [12, 25–29]. Oligodendrocyte development occurs over a relatively long period of time, from the embryonic stage to postnatal stage. Therefore, accurate processing of the time it takes for each stage of development is allowed during prolonged development. In our study, the expression pattern of PDGFRα and Olig2 in Cdk5−/− mice proved to be different from that of the Cdk5+/+ mice at embryonic day (E) 18 (Fig. 2). After calculating the number of stained cells, however, we could not detect any significant reduction in OPCs and undifferentiated oligodendrocytes in the cerebral cortices of Cdk5−/− mutant mice and littermate controls (Fig. 2e). Because Cdk5 plays a pivotal role in neuron migration, and the mutant mice show inverted layers in the cerebral cortex, different distribution OPCs may be a secondary consequence of the abnormal layer structure of Cdk5−/− mice. Taken together, the myelin defect in Emx1-cKO mice was not likely due to a failure to generate OPCs, but more likely due to the disturbance of the normal maturation program, as the OPCs were not reduced in Cdk5−/− mice. Moreover, the number of undifferentiated oligodendrocytes of Cdk5−/− mice was also the similar to those of the littermates of Cdk5+/+ mice.
Fig. 2.
In situ hybridization in the coronal sections from Cdk5+/+ and Cdk5−/− mouse embryos at E18 using Olig2 and PDGFRα probes. Coronal sections from the brains of Cdk5+/+ and Cdk5−/− mice embryos at E18 were hybridized with a PDGFRα (a, b) or Olig2 (c, d) probe. The staining shows that Cdk5−/− mice had different expression patterns of Olig2 in the cerebral cortex than the Cdk5+/+ mice did, but that the number of positive cells was not significantly different in Cdk5−/− compared to Cdk5+/+ mice (e). Scale bar: 500 μm
Expression Pattern of Immature and Mature Oligodendrocyte Markers in Brain Tissue of Emx1-cKO Mice
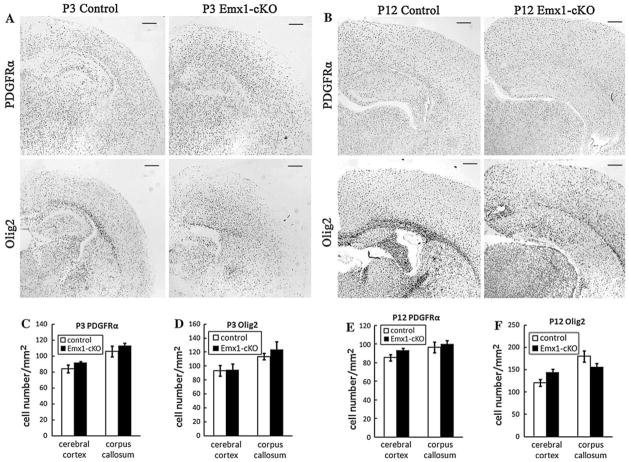
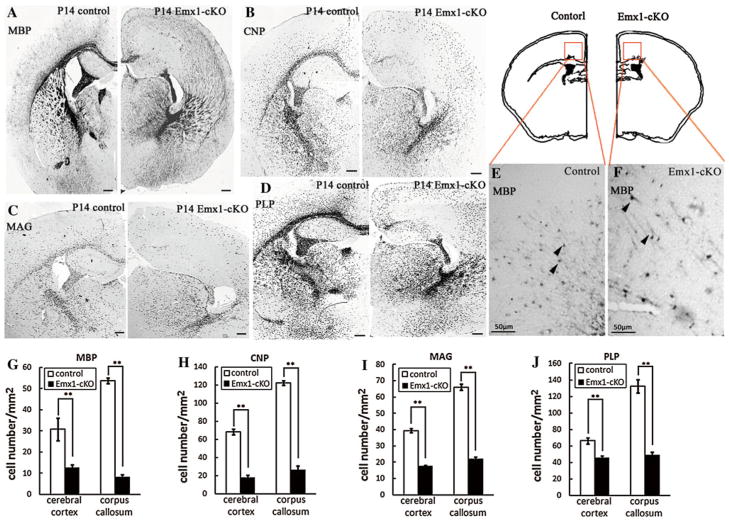
We examined the expression of OPC markers and myelinating oligodendrocyte markers to figure out which step was disturbed in Emx1-cKO mice. In situ hybridization was performed using the coronal cryosections of Emx1-cKO mice and their littermate controls. As a majority of oligodendrocytes do not start differentiation until postnatal day (P) 14, we selected three time points (P3, P12 and P14) to perform the in situ hybridization. We found that PDGFRα or Olig2 positive OPCs presented robustly in cerebral cortices and corpus callosums of both Emx1-cKO mice and their littermate controls at postnatal days 3 and 12. The number of PDGFRα or Olig2 positive OPCs in Emx1-cKO mouse brain sections was similar to that of their littermate controls (Fig. 3). In contrast to OPCs, the MBP positive myelinating oligodendrocytes were reduced in the corpus callosum and cerebral cortex of Emx1-cKO mice, compared to their littermate controls (Fig. 4a, g). The high magnification of in situ hybridization images (Fig. 4e, f) showed that the MBP positive signal was observed in the soma and the processes of oligodendrocytes in both control mice and Emx1-cKO mice. Distinct patterns of MBP signal distribution (Fig. 4a) and axonal trajectories (Figs. 5, 6) were observed in Emx1-cKO mice. Moreover, the number of cells labeled by CNP, MAG and PLP probes had a pattern that was consistent with that of the MBP of Emx1-cKO mice (Fig. 4b, c, d, h, i, j). Taken together, the number of OPCs and immature oligodendrocytes were the same in Emx1-cKO mice as in littermate controls. In contrast, the number of mature oligodendrocytes was distinctly reduced in Emx1-cKO mice compared with littermate controls. All these data indicate that the maturation of oligodendrocytes is likely to be disturbed at the postnatal stage in Emx1-cKO mice.
Fig. 3.
In situ hybridization in the coronal sections from littermate controls and Emx1-cKO mice at P3 and P12. Coronal sections from the brains of control and Emx1-cKO mice at P3 (a) and P12 (b) were hybridized with immature oligodendrocytes and OPCs markers (PDGFRα and Olig2 probes). The number of positive cells in the cerebral cortex and corpus callosum of Emx1-cKO mice was almost the same as it was with littermate control mice (c, d, e, f). Scale bar: 100 μm
Fig. 4.
In situ hybridization in the coronal sections from littermate controls and Emx1-cKO mice at P14. Coronal sections from the brains of control and Emx1-cKO mice at P14 were hybridized with differentiated oligodendrocyte markers [MBP (a), CNP (b), MAG (c) and PLP (d)]. The high magnification images (e, f) showed the MBP positive signal was not only in the soma but also in the processes of oligodendrocytes (arrowheads) in both control and Emx1-cKO mice. The number of stained cells in the corpus callosum and cerebral cortex was distinctly reduced in Emx1-cKO mice compared with littermate control mice (g, h, j, i). **P < 0.01. Scale bar in a, b, c and d: 200 μm; scale bar in e and f: 50 μm
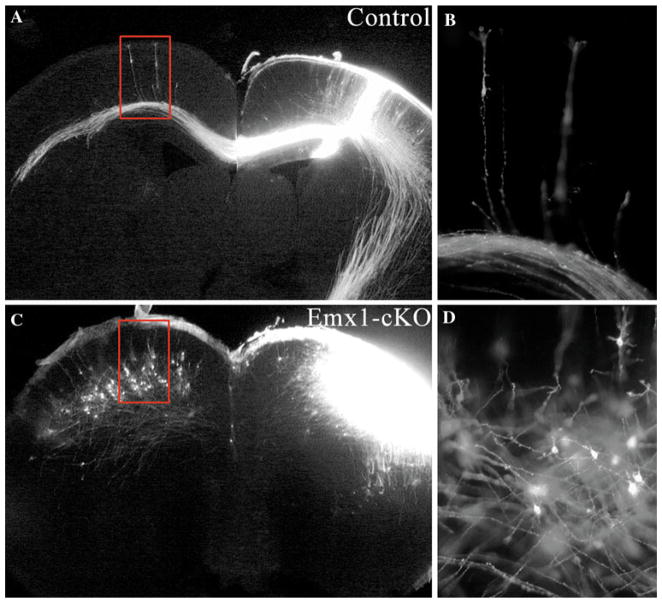
Fig. 5.
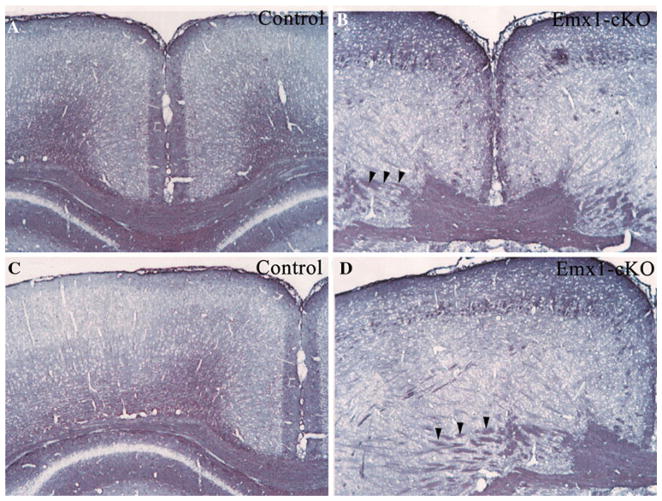
Axon trajectory of DiI labeling neuron in P0 Emx1-cKO and control brain cortices. DiI crystals were placed in the cerebral cortices of P0 Emx1-cKO (c, d) and its littermate controls (a, b). In control mice, cortical pyramidal neurons extended their apical dendrite toward the pia and their single axon down toward the ventricular zone (b). By contrast, in Emx1-cKO cortex, the neurons extend their dendrites in multiple directions, and axon run obliquely within cortices (d)
Fig. 6.
Neurofilament staining (SMI-31) of Emx1-cKO and control brain cortices at P0. SMI-31 generally reacts broadly with thick and thin axons and some dendrites but not soma of neuron. Commissural neurons extended their axons down to ventricular zone and formed corpus callosum in control mice (a, c), while in Emx1-cKO mice (b, d) the axon trajectory was abnormal and axons (arrowheads) run obliquely within cortex, in addition a short corpus callosum was observed in Emx1-cKO mice (b, d)
Hypomyelination in Emx1-cKO Mouse Brains
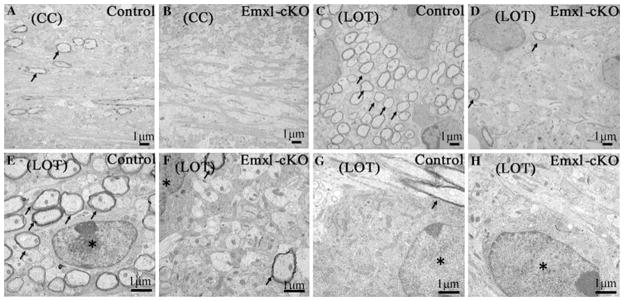
To further investigate the morphology of the myelin sheaths in Emx1-cKO mice, we examined lateral olfactory tract (LOT) and corpus callosum (CC) by electron micrograph. Multilamellar myelin sheaths were apparent around the axons in control mice, whereas in Emx1-cKO mice the axons were essentially unmyelinated (Fig. 7). We found that LOT from Emx1-cKO mice contained 97.5% less myelinated axons than littermate controls (Fig. 7c, d), while corpus callosum from Emx1-cKO mice contained no myelinated axons in the field of view compared with the littermate controls (Fig. 7a, b). On the transverse section of myelinated axons in LOT and CC, one oligodendrocyte was surrounded by many axons (Fig. 7e, g). These axons were wrapped by myelin sheaths, whereas in Emx1-cKO mice, very few axons were sheathed by myelin (Fig. 7f, h). All these data show that the myelin system was strongly reduced in Emx1-cKO mice, and may indicate that maturation is disturbed in Emx1-cKO mice.
Fig. 7.
Electron micrographs of LOT (lateral olfactory tract) and CC (corpus callosum) from control and Emx1-cKO mice at P14. Multilamellar myelin sheaths were apparent around the axons in WT mice (a, c), while the axons in Emx1-cKO mice were essentially unmyelinated (b, d). In LOT and CC of WT mice, the axons around the oligodendrocyte were wrapped in myelin sheaths (e, g), while in Emx1-cKO mice, only a few axons were sheathed by myelin (f, h). Arrow: myelinated axon, asterisk: oligodendrocytes
Lack of Cdk5 Impaired Oligodendrocyte Differentiation
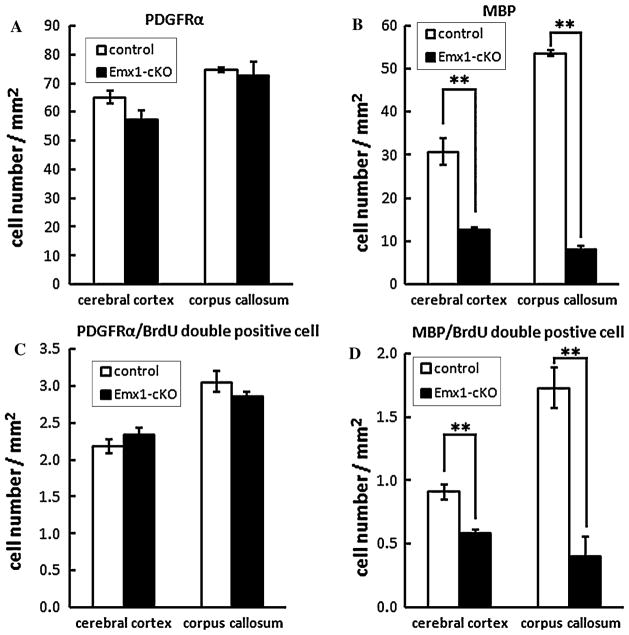
In light of the previous results, we have found that the number of Olig2 and PDGFRα labeled cells has no distinct difference between Emx1-cKO mice and their littermate controls, whereas the MBP, PLP, MAG and CNP labeled cells were substantially reduced in Emx1-cKO mice. We can infer that the differentiation of the oligodendrocytes was severely affected. To test this hypothesis, we injected the mutant mice and their littermate controls intraperitoneally with BrdU at P9, and sacrificed the mice to collect samples at P14. We performed in situ hybridization with PDGFRα/MBP probes and counted the cells labeled by BrdU and PDGFRα/MBP. The number of the MBP labeled cells was significantly reduced in both the cerebral cortex and the corpus callosum in Emx1-cKO mice (Fig. 8b), whereas the number of PDGFRα labeled cells in Emx1-cKO mice was nearly the same as it was in their littermate controls (Fig. 8a). These results were consistent with the previous ones, and the number of cells labeled with both MBP and BrdU was distinctly decreased in Emx1-cKO mice compared to littermate controls (Fig. 8d). The number of cells labeled with both PDGFRα and BrdU in Emx1-cKO mice was similar to the amount in littermate controls (Fig. 8c). This result supported our hypothesis that the differentiation was affected in the Emx1-cKO. Thus, our results imply that Cdk5 plays an important role in oligodendrocyte differentiation in vivo.
Fig. 8.
In situ hybridization with PDGFRα and MBP probes using a BrdU treated mice sample. The number of MBP labeled cells was significantly reduced in both the cortex and corpus callosum of Emx1-cKO mice (b) (**P < 0.01), whereas the number of PDGFRα in Emx1-cKO mice was nearly the same as that of their littermate controls (a). The number of cells labeled with both MBP and BrdU was distinctly decreased in Emx1-cKO mice compared to littermate controls (d) (**P < 0.01). The number of cells labeled with both PDGFRα and BrdU in Emx1-cKO mice was similar to that of littermate controls (c)
Discussion
In general, Cdk5 substrates are proteins involved in versatile functions, such as signal transduction, neuron migration, neuron differentiation and axonal elongation, and these substrates modulate cell morphology and motility [1–4, 31, 32]. Cdk5 is expressed in various types of cells [29], especially in the neuronal system. Its kinase activity does not only exist in neurons but also in microglia [19], in astrocytes [33] and in oligodendrocytes [17]. It has also been reported that Cdk5 is expressed in both precursor cells and oligodendrocytes; however, the activity of Cdk5 is nearly twofold higher in oligodendrocytes than in precursor cells [17]. This also indirectly supports our hypothesis that the deletion of Cdk5 in oligodendrocytes may induce more defects than deletion in precursor cells. There are very few reports regarding the function of Cdk5 in oligodendrocytes; the studies presented here provide evidence that Cdk5 also plays a critical role in the differentiation of oligodendrocytes in vivo. The Emx1-cKO mice showed that the number of differentiated oligodendrocytes decreased in comparison to the control mice. In addition, the result of electron microscopy showed that the axons of lateral olfactory tract and corpus callosum were essentially unmyelinated. As we reported, inverted cortical layers were observed in both Cdk5−/− and Emx1-cKO mice [6, 23]. It demonstrated that Cdk5 functions as an important factor in radial neuronal migration during corticogenesis. Furthermore, abnormal axonal trajectory was observed in Cdk5−/− mice [23]. As similar to Cdk5−/− mice, axons of cortical neurons did not run straight down to ventricular side but run obliquely within cortices in Emx1-cKO mice (Figs. 5, 6). A different localization pattern of OPCs and immature oligodendrocytes as well as mature oligodendrocytes observed in Emx1-cKO cortices may be a secondary consequence of the abnormal axonal trajectory observed in Emx1-cKO mice. Similar abnormalities in axonal trajectory and distribution of MBP-immunoreactivity were reported in reeler mice which have inverted laminar structure of cerebral cortex [34]. Importantly, in this paper, Mikoshiba et al., demonstrated that the timing of myelination in reeler was similar to that of control mice [34]. Abnormal axonal trajectory and normal myelination were also observed in Shaking rat Kawasaki [35] which shares reelin gene defect with reeler mice [36]. Therefore, defect of oligodendrocyte differentiation in Emx1-cKO mice we described here is not secondary consequence of abnormal trajectory of axon. Our results are consistent with the in vitro results [18], which showed that, when adding Cdk5 inhibitor rescovitine to the cultured oligodendrocytes, the cells cannot develop processes, unlike in the case of control mice. These results also indicated that paxillin (one of the Cdk5 substrates) plays an important role in the morphological changes of oligodendrocyte differentiation. Because phosphorylated paxillin provides the binding sites for other proteins, or modulates interaction between paxillin and other proteins [37], paxillin will only be one part of the signal transduction pathway that induces oligodendrocyte differentiation. In the central nervous system, oligodendrocytes’ maturation from process specification to terminal differentiation to the formation of a myelin sheath around the axons undergoes several stages. Signal transduction, transcription factors and microRNA [38–41] are all involved in modulating the oligodendrocytes’ differentiation. In this large interactive network, our studies may offer new insight into the mechanism of oligodendrocyte differentiation.
Acknowledgments
We wish to thank Drs. Itohara and Iwasato for providing the Emx1-cre mice, and Drs. Dragatsis and Zeitlin for providing the CamKII-cre mice. We would also like to thank Dr. Elias Utreras and Eric Contreras for critical reading of the manuscript.
Footnotes
Special Issue: In Honor of Dr. Mikoshiba.
Contributor Information
Xiaojuan He, Department of Life Science and Medical Bio-Science, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan.
Satoru Takahashi, Department of Pediatrics, Asahikawa Medical College, Asahikawa 078-8510, Japan.
Hiromi Suzuki, Laboratory for Developmental Neurobiology, Brain Science Institute, RIKEN, Saitama 351-0198, Japan.
Tsutomu Hashikawa, Support Unit for Neuromorphological Analysis, Brain Science Institute, RIKEN, Saitama 351-0198, Japan.
Ashok B. Kulkarni, Functional Genomics Section, Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA
Katsuhiko Mikoshiba, Laboratory for Developmental Neurobiology, Brain Science Institute, RIKEN, Saitama 351-0198, Japan.
Toshio Ohshima, Email: ohshima@waseda.jp, Department of Life Science and Medical Bio-Science, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan.
References
- 1.Dhavan R, Tsai LH. A decade of Cdk5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 2.Smith D. Cdk5 in neuroskeletal dynamics. Neurosignals. 2003;12:239–251. doi: 10.1159/000074626. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka T, Veeranna, Ohshima T, et al. Neuronal cyclin-dependent kinase 5 activity is critical for survival. Neuroscience. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant P, Sharma P, Pant HC, et al. Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur J Nerurosci. 2001;268:1534–1546. [PubMed] [Google Scholar]
- 5.Wu DC, Yu YP, Lee NTK, et al. The expression of Cdk5, p35, p39, and Cdk5 kinase activity in developing, adult, and aged rat brains. Neurochem Res. 2000;25:923–930. doi: 10.1023/a:1007544106645. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima T, Ward JM, Huh CG, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corti-cogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko J, Humbert S, Brorson T, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore E, Ohshima T, Goffinet A, et al. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paglini G, Caceres A. The role of the Cdk5-p35 kinase in neuronal development. Eur J Biochem. 2001;268:1528–1533. [PubMed] [Google Scholar]
- 10.Delalle I, Bhide PG, Caviness VS, et al. Temporal and spatial patterns of expression of p35, a regulatory subunit of cyclin-dependent kinase 5, in the nervous system of the mouse. J Neurocytol. 1997;26:283–296. doi: 10.1023/a:1018500617374. [DOI] [PubMed] [Google Scholar]
- 11.Lee JC, Mayer-Proschel M, Rao MS, et al. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–928. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 13.Dana M, McTigue DM, Richa B, et al. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 14.de Castro F, Bribián A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Rev. 2005;49:227–241. doi: 10.1016/j.brainresrev.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang XM, Strocchi P, Cambi F. Changes in the activity of cdk2 and cdk5 accompany differentiation of rat primary oligodendrocytes. J Cell Biochem. 1998;68:128–137. doi: 10.1002/(sici)1097-4644(19980101)68:1<128::aid-jcb13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto Y, Yamauchi J, Chan JR, et al. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci. 2007;120:4355–4366. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Ohshima T, Hirasawa M, et al. Conditional deletion of neuronal cyclin-dependent kinase 5 in developing forebrain results in microglial activation and neurodegeneration. Am J Pathol. 2010;176:320–329. doi: 10.2353/ajpath.2010.081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorski JA, Talley T, Qiu M. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emxl-expressing lineage. J Neurosci. 2002;22(15):6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirasawa M, Ohshima T, Takahashi S, et al. Perinatal abrogation of Cdk5 expression in brain results in neuronal migration defects. Proc Natl Acad Sci USA. 2004;101:6249–6254. doi: 10.1073/pnas.0307322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasato T, Datwani A, Wolf AM, et al. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshima T, Hirasawa M, Tabata H, et al. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134:2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- 24.Araya R, Kudo M, Kawano M, et al. BMP signaling through BMPRIA in astrocytes is essential for proper cerebral angiogenesis and formation of the blood-brain-barrier. Mol Cell Neurosci. 2008;38:417–430. doi: 10.1016/j.mcn.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billon N, Jolicoeur C, Tokumoto Y, et al. Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TR#1) EMBO J. 2002;21:6452–6460. doi: 10.1093/emboj/cdf662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nancy EJ, Berman J, Johnson K, et al. Early generation of glia in the intermediate zone of the developing cerebral cortex. Dev Brain Res. 1997;101:149–164. doi: 10.1016/s0165-3806(97)00060-6. [DOI] [PubMed] [Google Scholar]
- 27.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 28.Ventura RE, Goldman JE. Telencephalic oligodendrocytes battle it out. Nat Neursci. 2006;9:153–154. doi: 10.1038/nn0206-153. [DOI] [PubMed] [Google Scholar]
- 29.Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 30.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 31.Johansson JU, Lilja L, Chen XL, et al. Cyclin-dependent kinase 5 activators p35 and p39 facilitate formation of functional synapses. Mol Brain Res. 2005;138:215–227. doi: 10.1016/j.molbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao C, Negash S, Wang HS. Cdk5 mediates changes in morphology and promotes apoptosis of astrocytoma cells in response to heat shock. J Cell Sci. 2001;114:1145–1154. doi: 10.1242/jcs.114.6.1145. [DOI] [PubMed] [Google Scholar]
- 34.Mikoshiba K, Takamatsu K, Tsukada Y. Altered myelinated fiber trajectory at various postnatal days in the cerebral cortex of reeler mice by immunohistochemical stain with MBP antiserum. Dev Neurosci. 1985;4:199–205. doi: 10.1159/000112288. [DOI] [PubMed] [Google Scholar]
- 35.Aikawa H, Nonaka I, Woo M, et al. Shaking rat Kawasaki (SRK): a new neurological mutant rat in the Wistar strain. Acta Neuropathol. 1988;76(4):366–372. doi: 10.1007/BF00686973. [DOI] [PubMed] [Google Scholar]
- 36.Kikkawa S, Yamamoto T, Misaki K, et al. Missplicing resulting from a short deletion in the reelin gene causes reeler-like neuronal disorders in the mutant shaking rat Kawasaki. J Comp Neurol. 2003;463(3):303–315. doi: 10.1002/cne.10761. [DOI] [PubMed] [Google Scholar]
- 37.Turner CE. Paxillin interactions. J Cell Sci. 2000;113:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 38.Wegner M. A matter of identity: transcriptional control in oligodendrocytes. J Mol Neurosci. 2008;35:3–12. doi: 10.1007/s12031-007-9008-8. [DOI] [PubMed] [Google Scholar]
- 39.Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–1299. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- 40.Zhao XH, He XL, Han XL, et al. MicroRNA-mediated control of oligodendrocytes differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dugas JC, Cuellar TL, Scholze A, et al. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]