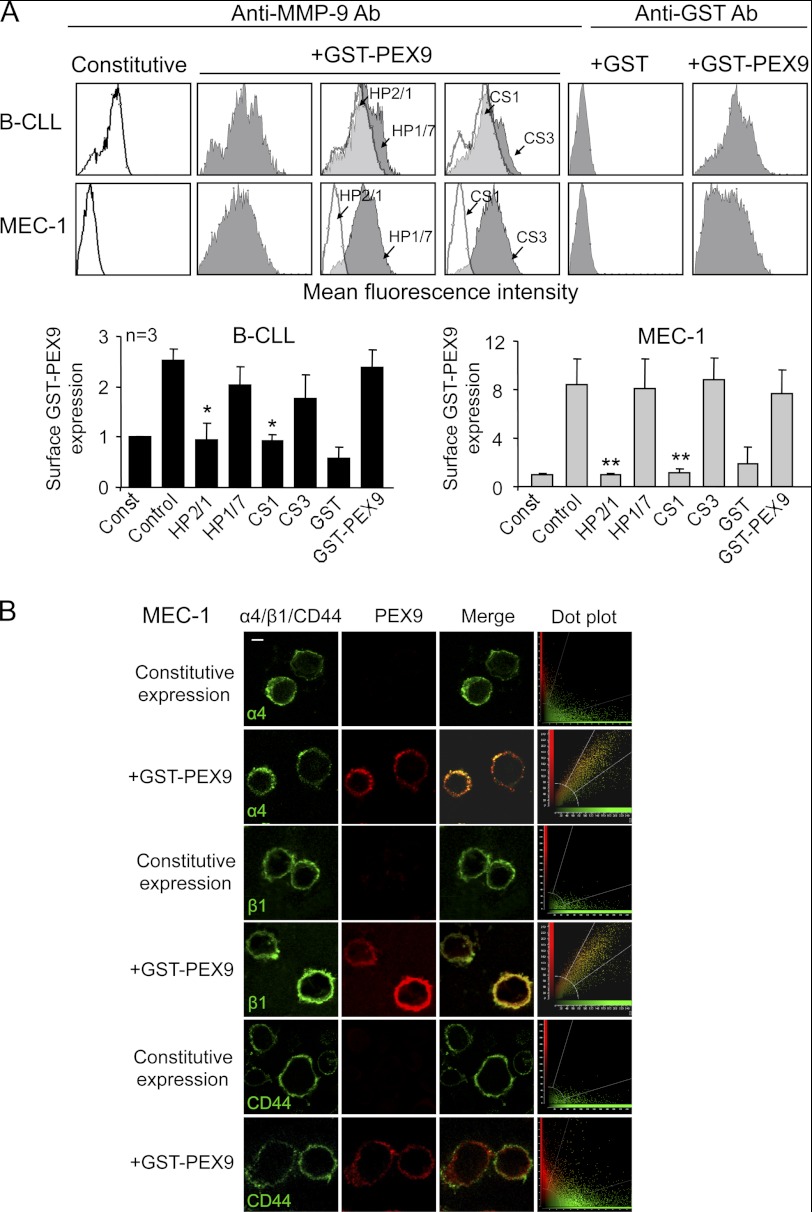
FIGURE 2.
α4β1 integrin mediates binding of soluble GST-PEX9 to B-CLL cells. A, primary B-CLL cells (patients 1, 2, 11) and MEC-1 cells (3 different experiments) with or without previous incubation with the indicated Abs or peptides were incubated for 30 min with or without GST-PEX9 (0.4 or 0.8 μm for B-CLL or MEC-1 cells, respectively) and analyzed by flow cytometry using anti-MMP-9 or anti-GST Abs. A representative B-CLL sample (patient 1) out of the three studied is shown. White areas represent constitutive (pro)MMP-9 expression; gray areas, (pro)MMP-9 expression upon GST-PEX9 binding. Constitutive MMP-9 expression was normalized to 1, and average quantitative values are shown. *, p ≤ 0.05; **, p ≤ 0.01. B, MEC-1 cells with or without previous incubation with 0.6 μm GST-PEX9 were added to glass coverslips coated with 10 μg/ml poly-lysine Lys. After 1 h at 37 °C, cells were fixed and analyzed by confocal microscopy using specific Abs for α4, β1, CD44, or MMP-9 followed by Alexa488- or Alexa568-labeled secondary Abs. Colocalization of GST-PEX9 with α4β1 integrin was further demonstrated using dot-plot analyses. The bar represents 5 μm.