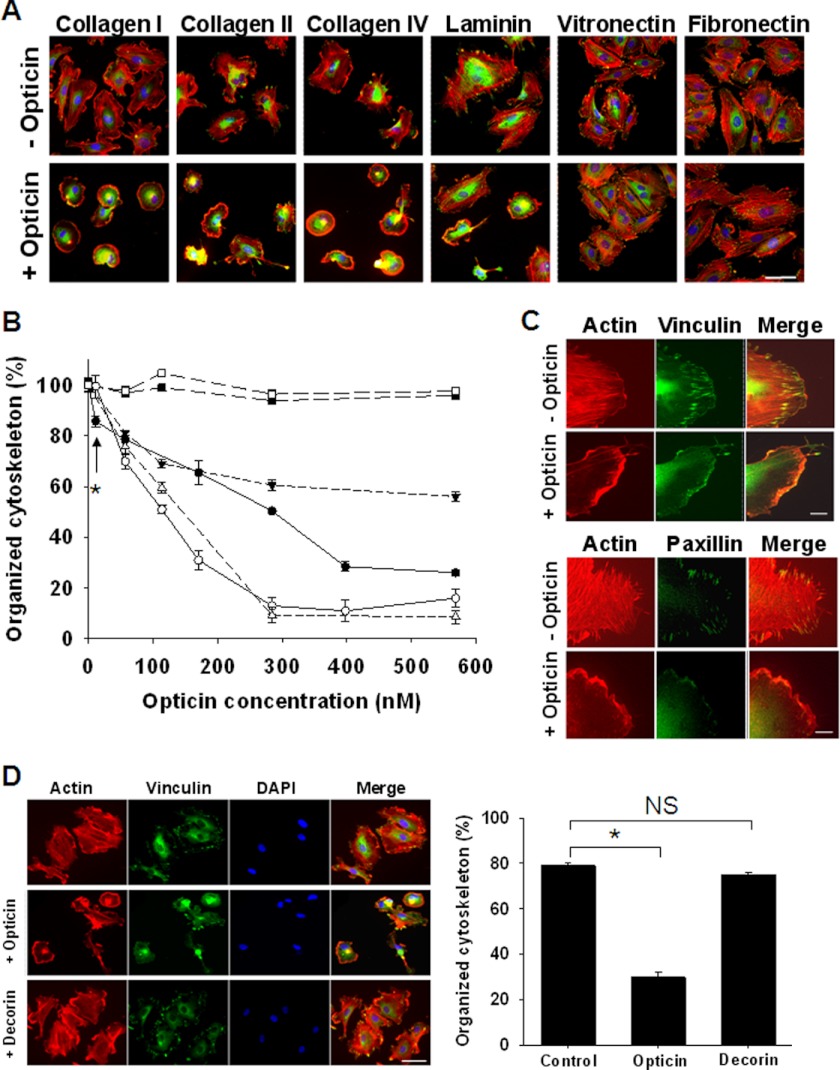
FIGURE 4.
Opticin disrupts HUVEC morphology when spread on collagen types I, II, and IV and laminin. A, opticin was added at 250 nm in solution to HUVECs spread on plates coated with collagen types I, II, and IV, and laminin, vitronectin, or fibronectin. Visualization of stress fibers (actin in red), focal adhesions (vinculin in green), and nuclei (in blue) showed that in absence of opticin, HUVECs spread on all these matrices; focal adhesions and actin stress fibers were clearly visible (top panel). However, after exposure to opticin the cells retracted: the stress fibers and focal adhesion disappeared when spread on collagen types I, II, and IV and laminin (bottom panel). The cell morphology was unaltered by opticin on vitronectin or fibronectin (scale bar, 50 μm). B, quantification showed that opticin disrupted HUVEC cytoskeleton in a concentration-dependent manner on collagen type I (●), collagen type II (○), collagen type IV (Δ), and laminin (▾) but not on vitronectin (■) or fibronectin (□); significant effects were observed at concentrations as low as 10 nm on collagen type I (*, p = 0.017). C, visualization of actin stress fibers (in red) and focal adhesions (vinculin and paxillin in green) showed that the discrete localization of both vinculin (top panel) and paxillin (bottom panel) in focal adhesions was lost when HUVEC spread on collagen type I were exposed to opticin at 250 nm (scale bar, 10 μm). D, when the effects of opticin and another SLRP family member, decorin, were compared, decorin had no effect, whereas opticin significantly disrupted HUVEC morphology (*, p < 0.001; not significant (NS), p = 0.287; scale bar, 50 μm).