FIGURE 5.
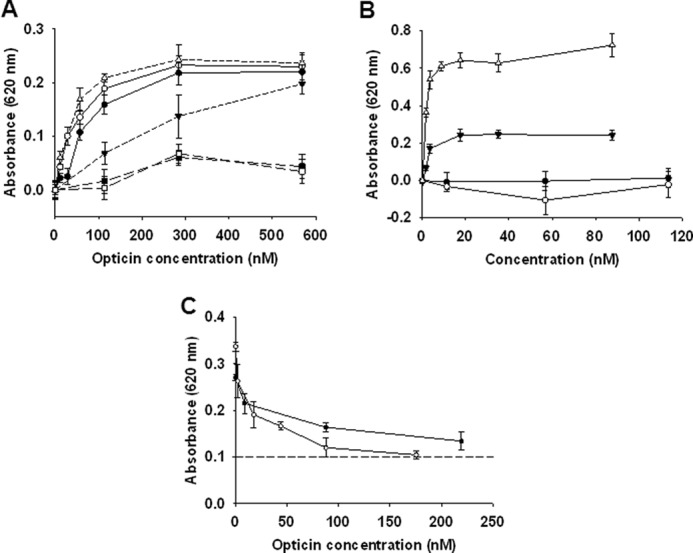
Opticin inhibits interactions between integrins and collagens/laminin. A, binding studies between opticin and various substrates revealed that it interacted strongly with collagen type I (●), collagen type II (○), and laminin (Δ), and more weakly to collagen type IV (▾). However, no binding was observed to vitronectin (■) or fibronectin (□). B, binding assays showed that both α1β1 and α2β1 integrins interacted with collagen type I strongly (▾ and Δ, respectively), but no binding to opticin was observed for either integrins (● and ○, respectively). C, inhibition studies using a fixed concentration of collagen type I preincubated with increasing opticin concentrations revealed that opticin inhibited collagen type I binding to both α1β1 and α2β1 integrins (● and ○, respectively). This inhibition was significant for both integrins at opticin concentrations as low as 1.75 nm (p = 0.02 and p = 0.002 for α1β1 and α2β1 integrins, respectively), corresponding to a 1:1 molar ratio between collagen type I and opticin; background absorbance level is indicated by a dashed line.
