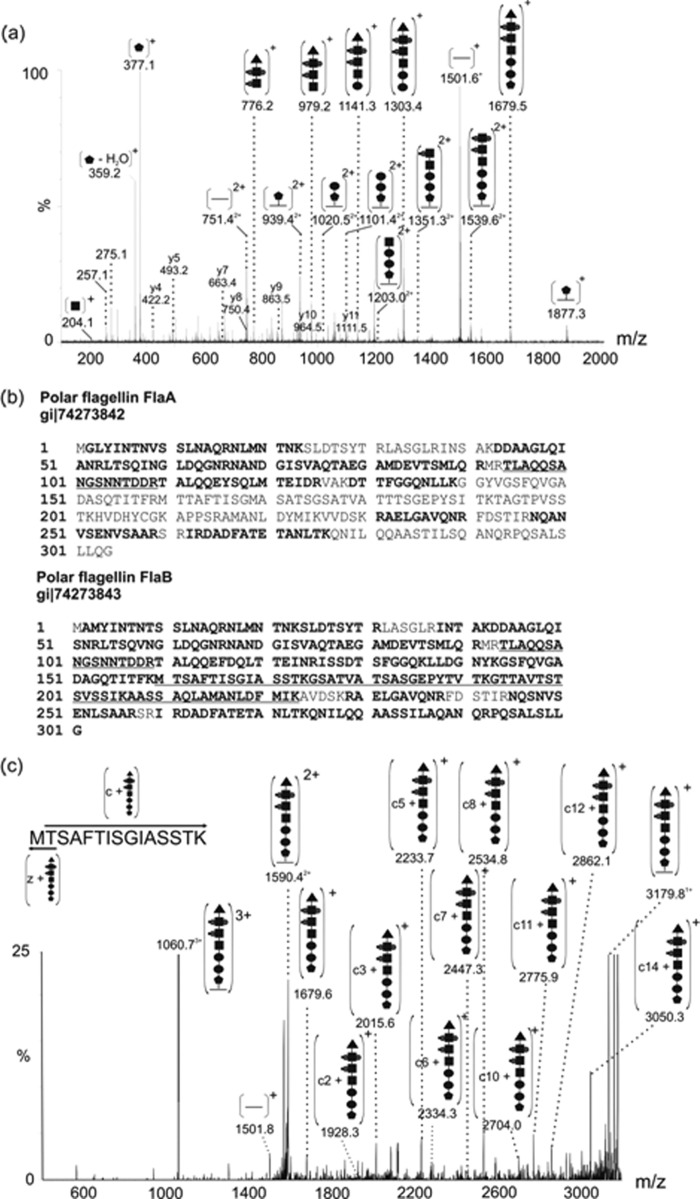
FIGURE 3.
MS/MS analysis of modified tryptic peptides from polar flagellar protein of A. hydrophila AH-3.
a, nLC-MS/MS spectrum of the triply protonated glycopeptide ion at m/z 1060.7 from polar flagellin. Visible were peptide fragment ions of low intensity, giving the peptide sequence 160TMTSAFTISGIASSTK174. Also observed was a daughter ion corresponding to the singly charged unmodified peptide ion (m/z 1501.1). The observed mass excess was 1678 Da, and a putative glycan oxonium ion was observed at m/z 1679. Indicated in the MS/MS spectrum are a series of neural losses from the glycan oxonium ion, giving a monosaccharide sequence on 376-162-162-203-297-377-102. It is likely that masses of 203 and 162 Da correspond to N-acetylhexosamine and hexose, respectively. Dominating the low mass region of the peptide MS/MS spectrum were glycan-related ions, at m/z 377 and 359. In addition, a doubly charged ion series was also observed, corresponding to glycopeptide fragments. b, protein sequence coverage from mass spectrometry analyses of flagellin proteins FlaA and FlaB are shown. Indicated in boldface type are unmodified peptides; boldface and underlined type indicates peptide modified with glycan. c, the triply charged glycopeptide ion at m/z 1060.7 was targeted for ETD with a reaction time of 300 s. In the resulting spectrum, the singly charged ion at m/z 1928.2 (c2 + glycan) along with ions corresponding to c3, c5, c6, c7, c8, c10, c11, c12, c14 all with the glycan still attached suggest that the peptide is modified at the threonine in position 161 of the protein sequence in O-linkage.  , 376-Da sugar; ●, hexose; ■, HexNAc; ▴, 102-Da unknown moiety; •, phosphorylation; ■, methylation.
, 376-Da sugar; ●, hexose; ■, HexNAc; ▴, 102-Da unknown moiety; •, phosphorylation; ■, methylation.