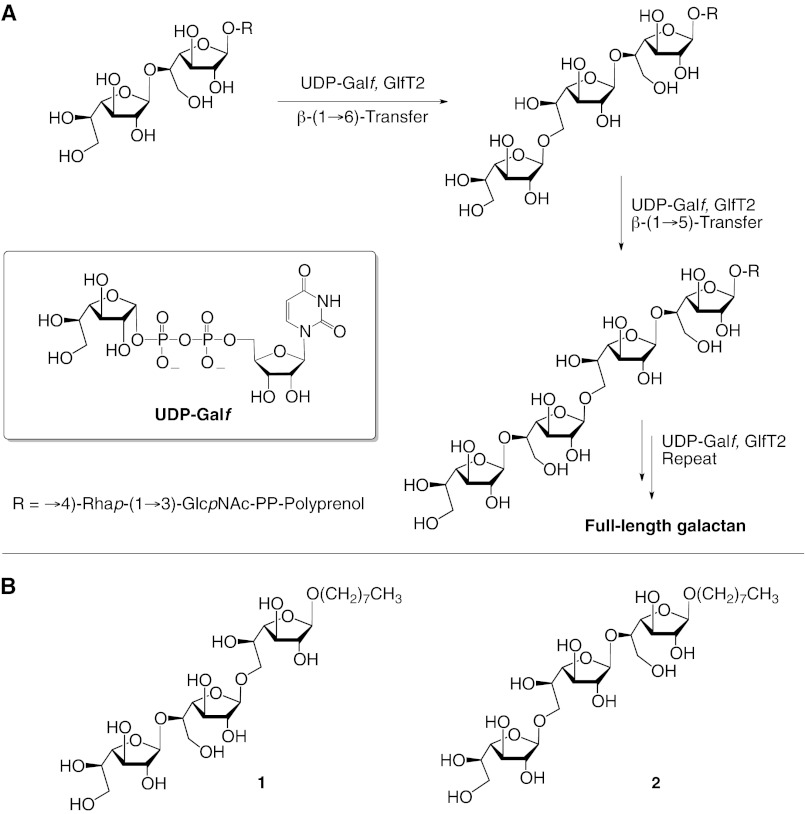
FIGURE 1.
A, alternating glycosylation reactions catalyzed by GlfT2. The β-(1→5)-transferase activity of the enzyme first glycosylates the galactofuranosyl residue at the nonreducing terminus of the polyprenol-bound tetrasaccharide acceptor. The resulting product is then a substrate for the β-(1→6)-transferase activity of GlfT2. Repetition of these two alternating glycosylations generates the elongated product. B, chemical structures of synthetic acceptor 1 and 2 used in enzyme kinetics and modeling studies.