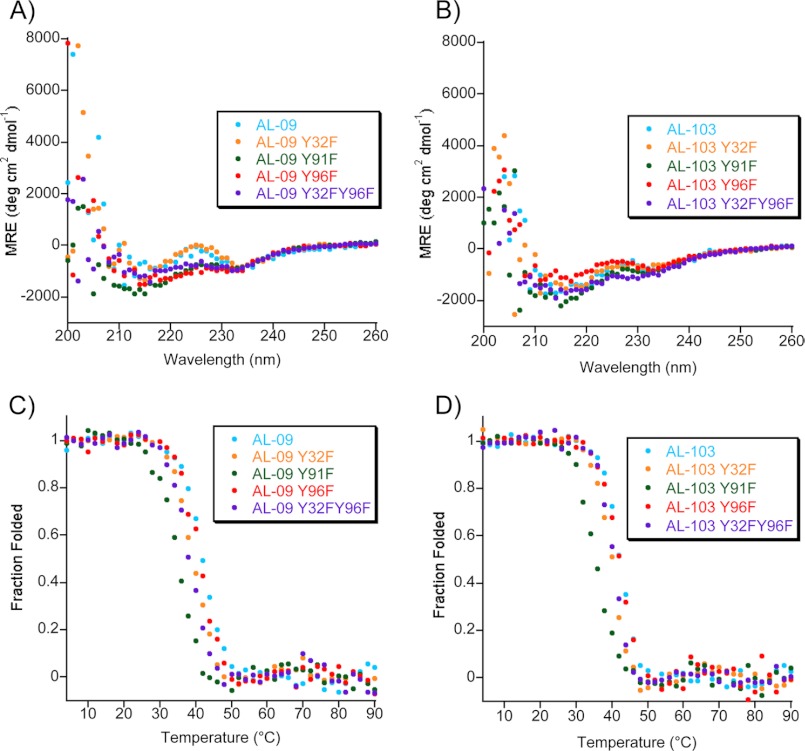
FIGURE 2.
Far UV-CD spectra of AL-09 Tyr-to-Phe mutants (A) and AL-103 Tyr-to-Phe mutants (B) display a β-sheet structure with two minima (∼235 and ∼216 nm). Experimental conditions were as follows: 20 μm protein in 10 mm Tris-HCl, pH 7.4, at 4 °C. Thermal denaturation of AL-09 Tyr-to-Phe mutants (C) and AL-103 Tyr-to-Phe mutants (D) show similar thermodynamic stability. Experimental conditions were as follows: 20 μm protein in 10 mm Tris-HCl, pH 7.4, from 4–90 °C at a rate of 30 °C per hour at the minimum wavelength between 215–218 nm, indicative of β-sheet structure; all display reversible thermal denaturation (not shown).