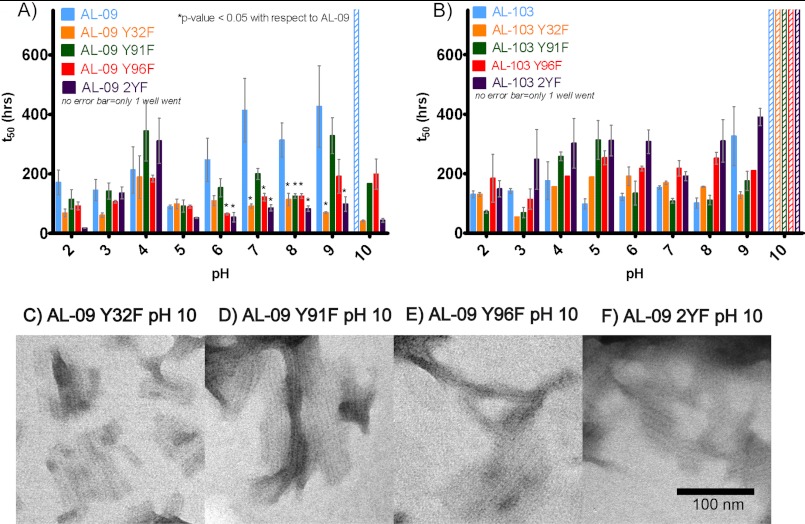
FIGURE 3.
Shown are fibril formation studies of AL-09 Tyr-to-Phe mutants (A) and AL-103 Tyr-to-Phe mutants (B) compared with AL-09 and AL-103, respectively. The t50 value, or the time at which the fibril formation reaction is 50% complete, was calculated by linear regression of the exponential phase of the fibril formation reactions (mean ± S.E.). All AL-09 Tyr-to-Phe mutants form fibrils readily at pH 2–10, whereas AL-09 does not form fibrils at pH 10. AL-103 and AL-103 Tyr-to-Phe mutants form fibrils at pH 2–9 with similar t50 values. Absence of an error bar indicates that only one of three reactions increased to 4-fold thioflavin T fluorescence. Electron microscopy of fibrils at pH 10 at the following time points: C, AL-09 Y32F: 240 h; D, AL-09 Y91F: 325 h; E, AL-09 Y96F: 265 h; F, AL-09 2YF: 255 h.