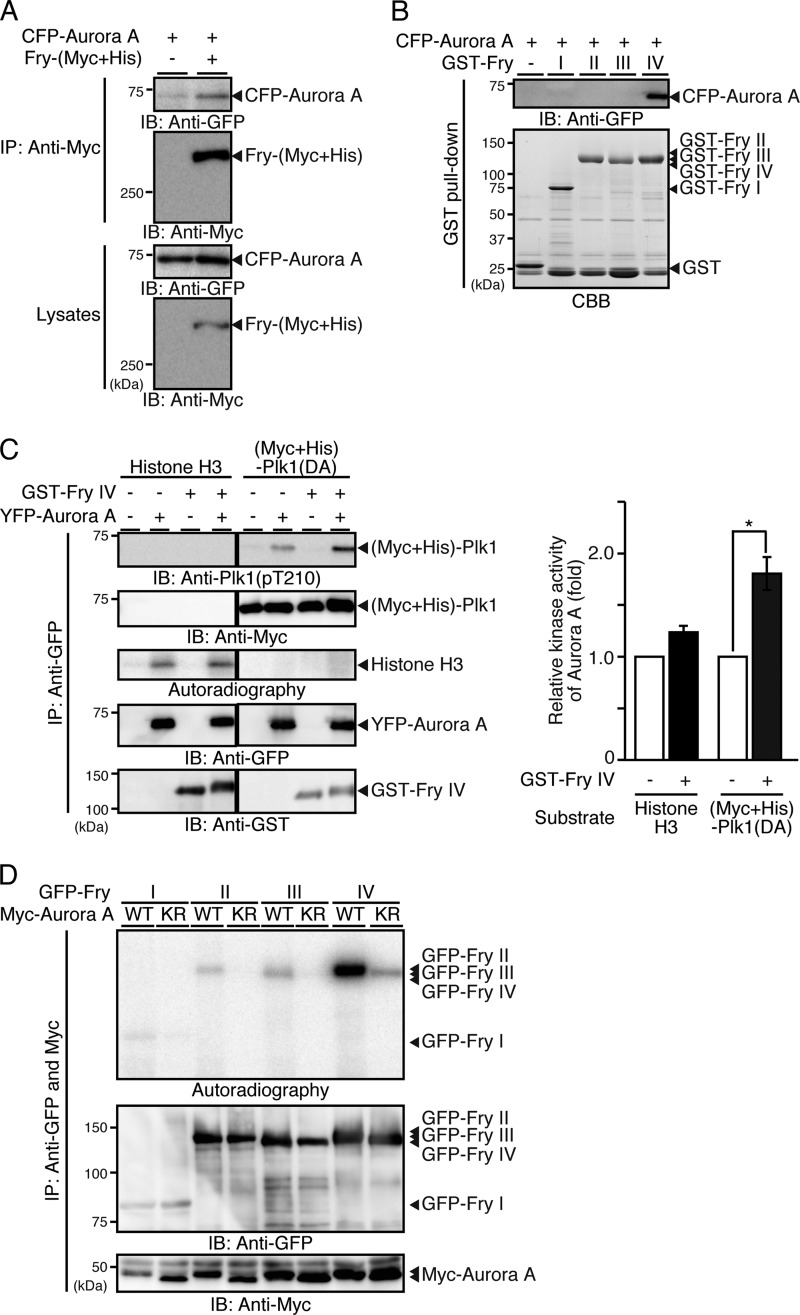
FIGURE 6.
Fry binds to Aurora A and promotes Aurora A-mediated Plk1 activation. A, Fry binds to Aurora A. HeLa cells were cotransfected with Fry-(Myc+His) and CFP-Aurora A and synchronized in early mitosis with thymidine-nocodazole. Cell lysates were immunoprecipitated (IP) with anti-Myc antibody and analyzed by immunoblotting (IB). B, Fry binds to Aurora A through the C-terminal region. Cell lysates expressing CFP-Aurora A were subjected to GST pull-down assays using GST-Fry fragments. Precipitates were analyzed by anti-GFP immunoblotting. C, Fry-IV enhances Aurora A-mediated Plk1 phosphorylation. 293T cells were transfected with YFP-Aurora A and synchronized in early mitosis with nocodazole. YFP-Aurora A was immunoprecipitated with anti-GFP antibody and subjected to in vitro kinase assays using an equal amount (1 μg) of purified (Myc+His)-Plk1(DA) or histone H3 as a substrate in the presence or absence of GST-Fry-IV. Histograms indicate the relative kinase activity of Aurora A toward Plk1(DA) or histone H3 with the Aurora A activity in the absence of GST-Fry-IV set to 1.0. Data are the means ± S.E. of triplicate experiments. *, p < 0.05. D, Aurora A phosphorylates the C-terminal region of Fry. 293T cell lysates transfected with Myc-Aurora A (WT or K162R) were mixed with lysates expressing GFP-Fry fragments, coprecipitated with anti-Myc and anti-GFP antibodies, and then subjected to in vitro kinase assay.