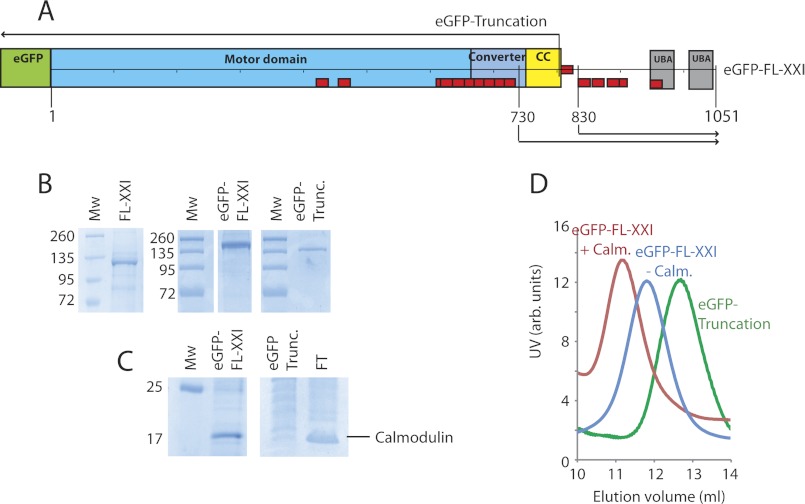
FIGURE 1.
Myosin-XXI constructs and protein purification. A, schematic of eGFP-tagged, full-length myosin-XXI. Other constructs, the eGFP-tagged truncated motor, as well as the 730 and 830 tail constructs are marked. Domains are the motor domain, converter region, predicted coiled coil (CC), and UBA. Red boxes are predicted calmodulin-binding motifs (see Fig. 4A). B, SDS-PAGE gel of full-length and truncated myosin-XXI coexpressed with calmodulin. The gel confirms clean purified protein with very little impurities (size markers in kDa). Mw, molecular weight. C, SDS-PAGE gels show calmodulin bound to the full-length but not to the truncated myosin construct. Calmodulin (untagged) was present in the flow-through (FT) created from the first purification stage where coexpressed proteins are bound to the Ni-Sepharose column. D, Superdextm-200 gel filtration of full-length myosin-XXI expressed in the presence of calmodulin (red), without calmodulin (blue), and of the truncated construct expressed in the presence of calmodulin (green). The elution volume increases with decreasing Stokes radius of the protein complex. All experiments were repeated at least three times for three separate protein purifications. Protein concentrations were between 5–8 μm depending on the preparation.