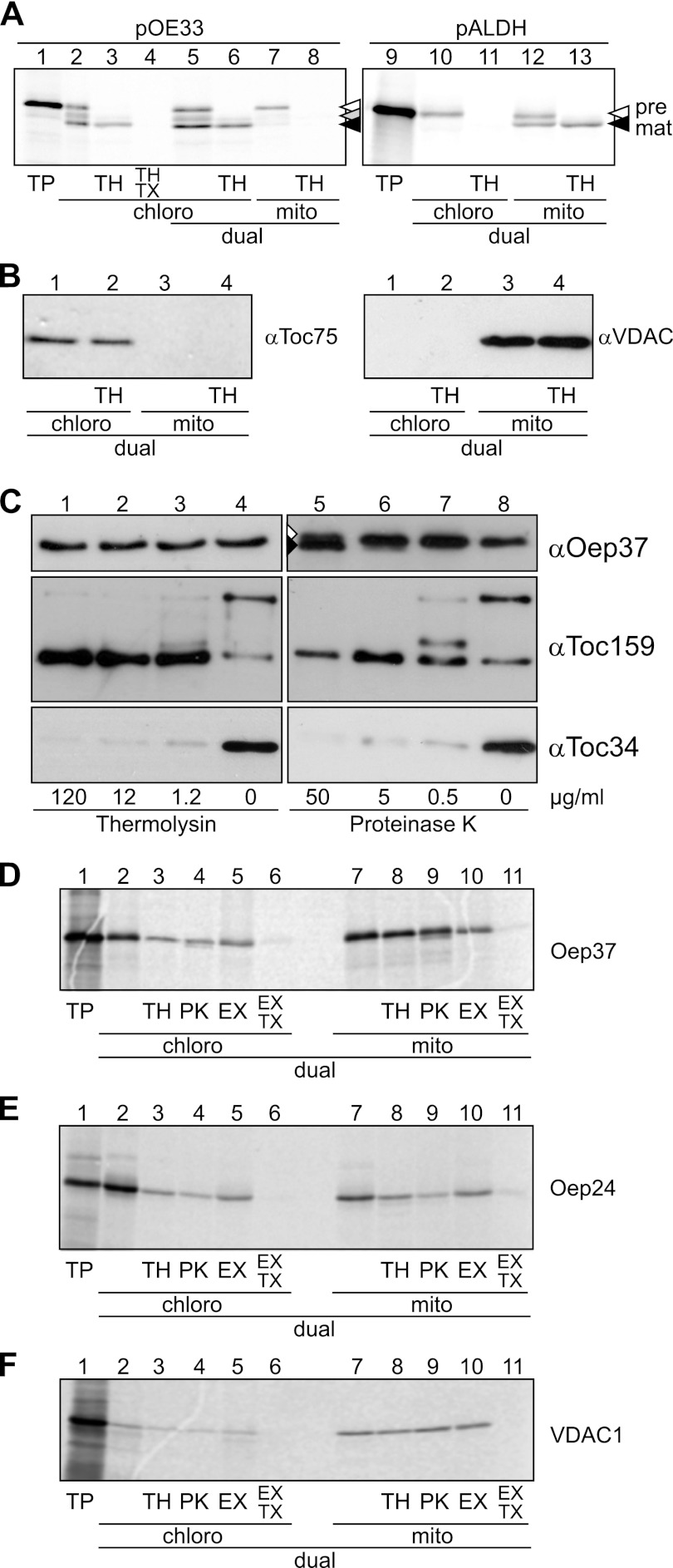
FIGURE 4.
Oep37 and Oep24 are targeted in vitro in a dual system to the OM of mitochondria and chloroplasts. A, single (lanes 2–4) and dual (lanes 5–13) import of rabbit reticulocyte-translated pOE33 or pALDH into isolated pea chloroplasts (chloro) (lanes 2–6, 10, and 11) and mitochondria (mito) (lanes 7 and 8 and 12 and 13). Translation products (TP, 10%) as input control are shown in lanes 1 and 9. Nonimported proteins were removed by thermolysin (TH, lanes 3, 6, 8, 11, and 13). In addition, 1% (v/v) Triton X-100 was added to one sample (TX, lane 4). Precursor and mature forms of pOE33 and pALDH are indicated by white and black arrowheads, respectively. The stromal intermediate form of pOE33 is indicated with a gray arrowhead. B, purity of the chloroplasts (lanes 1 and 2) and mitochondrial (lanes 3 and 4) fractions after dual import and re-purification of the organelles was assayed by Western blotting with the indicated organelle-specific antibodies. C, chloroplast outer envelope membranes were treated with the indicated concentrations of either thermolysin (lanes 1–4) or proteinase K (lanes 5–8). The membranes were then assayed by Western blotting using the indicated organelle-specific antibodies. Oep37 was partially sensitive to high amounts of proteinase K, and in addition to full-length protein, a slightly smaller degradation product was also observed (lane 5, white and black arrowheads, respectively). D–F, dual import of radiolabeled Oep37 (D), Oep24 (E), and VDAC1 (F) into chloroplasts and mitochondria (lanes 2–11). Translation products (TP, 10%) as input control are shown in lane 1. Nonimported proteins were removed by either thermolysin (TH, lanes 3 and 8) or proteinase K (PK, lanes 4 and 9) treatment. Full integration into the membrane was assayed by carbonate extraction (EX) in the presence or absence of Triton X-100 (TX, lanes 5, 6, 10, and 11).