FIGURE 5.
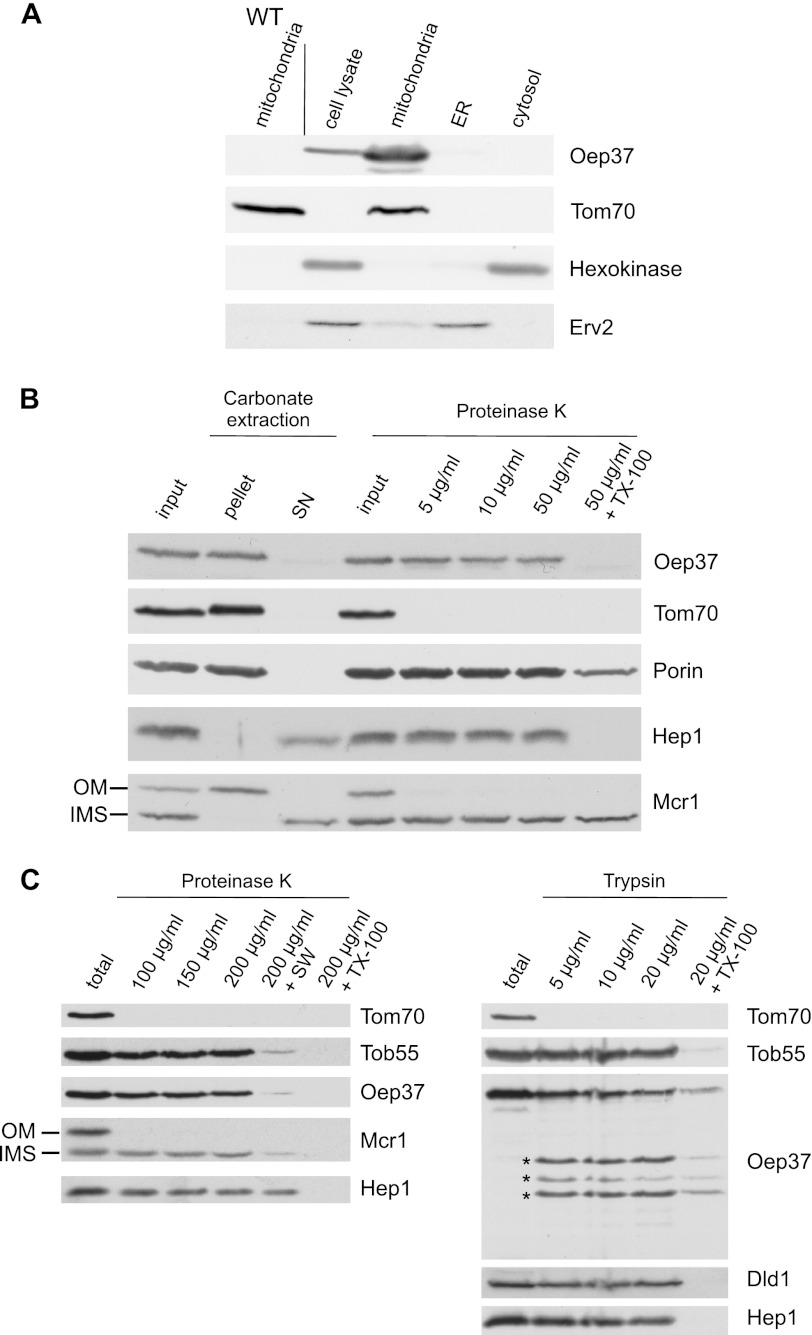
Oep37 expressed in yeast cells is assembled into the mitochondrial OM in a native conformation. A, Oep37 is located in mitochondria. Lysates of Oep37-expressing cells and fractions corresponding to mitochondria, endoplasmic reticulum (ER), and cytosol were analyzed by SDS-PAGE and immunodecoration with antibodies against Oep37, the mitochondrial protein Tom70, a marker protein for the cytosol (hexokinase), and the endoplasmic reticulum protein Erv2. Mitochondria isolated from untransformed WT cells were co-analyzed as a control. B, mitochondria isolated from cells expressing Oep37 were analyzed directly by SDS-PAGE (input) or were subjected first to carbonate extraction and then centrifuged to discriminate between membrane proteins in the pellet and soluble proteins in the supernatant (SN). Additional aliquots of mitochondria were left intact or were treated with the indicated amounts of proteinase K. Proteins were analyzed by SDS-PAGE and immunodecorated with antibodies against the indicated proteins as follows: Tom70, an OM protein exposed to the cytosol; Porin, a protein embedded in the OM; Hep1, a mitochondrial soluble matrix protein; Mcr1, a protein with two isoforms, a 34-kDa species exposed on the OM and a 32-kDa soluble one in the IMS. C, mitochondria isolated from cells expressing Oep37 were left intact (total) or were treated with the indicated amounts of proteinase K (left panel) or trypsin (right panel). In one sample, the mitochondria were swelled (+SW) before the treatment with PK. Proteins were analyzed by SDS-PAGE and immunodecorated with antibodies against the indicated proteins. Tob55, a protein embedded in the OM; Dld1, an inner membrane protein exposed to the IMS. Proteolytic fragments are indicated with an asterisk.