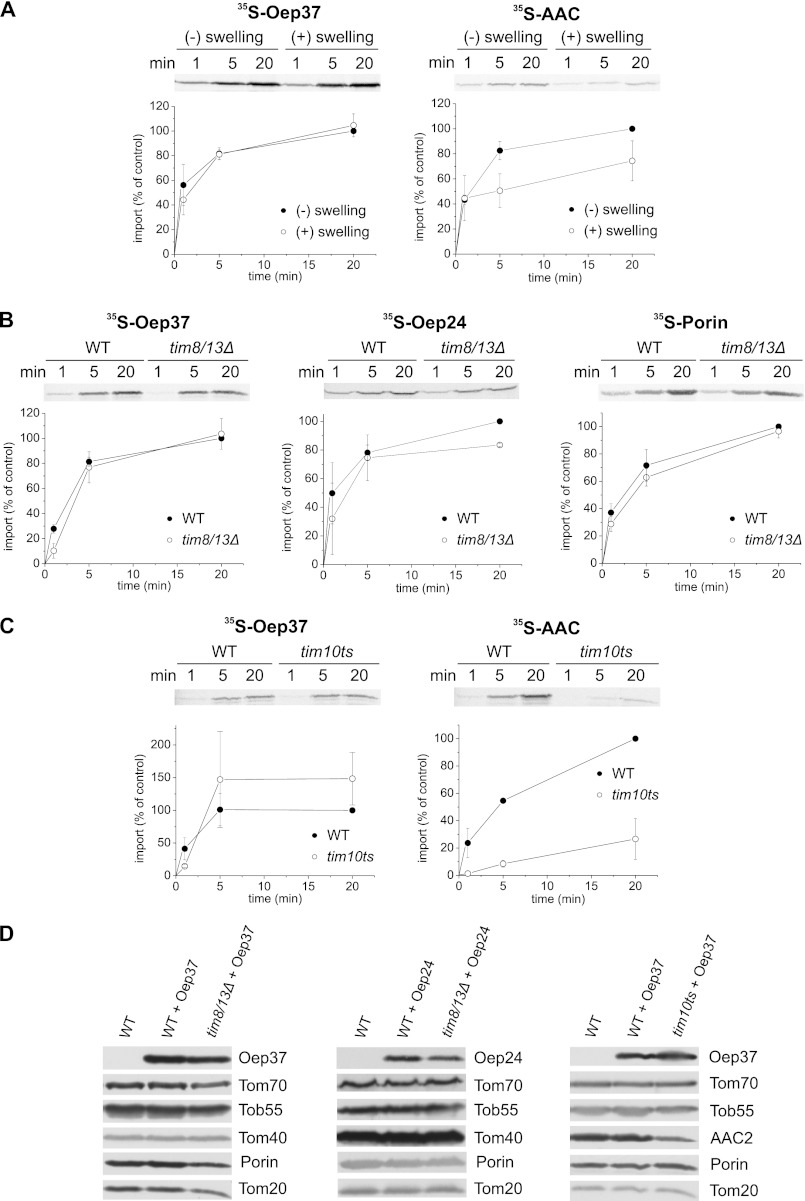
FIGURE 9.
Small Tim chaperones have only a minor role in the assembly of Oep37 and Oep24 into the mitochondrial OM. A, rupturing of the outer membrane does not compromise the assembly of Oep37. Radiolabeled precursors of Oep37 and AAC (as a control) were incubated for the indicated time periods with isolated intact mitochondria or with mitochondria that had been subjected to osmotic swelling. After import, mitochondria were pelleted and subjected to alkaline extraction, and the pellet fractions were analyzed by SDS-PAGE followed by autoradiography. The amount of precursor proteins imported into intact mitochondria for 20 min was set to 100%. An autoradiographic representative of three independent repeats and quantification of three independent experiments are presented. B, insertion of Oep37 and Oep24 is hardly affected in mitochondria lacking the Tim8/Tim13 complex. Radiolabeled precursors of Oep37, Oep24, and Porin were imported into mitochondria isolated from either tim8Δ/tim13Δ or its corresponding parental strain. Imported proteins were analyzed and quantified as described in the legends to Fig. 7A. C, insertion of Oep37 is not affected in mitochondria mutated in TIM10. Radiolabeled precursors of Oep37 and AAC were imported into mitochondria isolated from a strain harboring a temperature-sensitive allele of TIM10 (TIM10-1 (58)) or from its corresponding parental strain. Imported proteins were analyzed and quantified as described in the legends to Fig. 7A. D, mitochondria isolated from nontransformed WT cells and those isolated from either tim8/tim13Δ or TIM10-1 and their corresponding parental strains transformed with Oep37- or Oep24-encoding plasmid were analyzed by SDS-PAGE and immunodecoration with antibodies against Oep37 or Oep24, respectively. In addition, immunodecoration with antibodies against the indicated mitochondrial proteins was performed.