Background: A cross-talk between a kinase and phosphatase plays a critical role in determining cellular fate.
Results: We report that p38γ phosphorylates its phosphatase PTPH1 in regulation of Ras oncogenesis and stress response.
Conclusion: These results indicate that a MAPK can signal through its phosphatase.
Significance: These studies reveal a novel mechanism by which a MAPK signals through its phosphatase to determine cellular outcomes.
Keywords: p38 MAPK, Phosphatase, Ras, Stress, Transformation, Ras Oncogenesis, p38 MAPK, Phosphorylation, Protein-tyrosine Phosphatase, Stress Response
Abstract
Phosphatase plays a crucial role in determining cellular fate by inactivating its substrate kinase, but it is not known whether a kinase can vice versa phosphorylate its phosphatase to execute this function. Protein-tyrosine phosphatase H1 (PTPH1) is a specific phosphatase of p38γ mitogen-activated protein kinase (MAPK) through PDZ binding, and here, we show that p38γ is also a PTPH1 kinase through which it executes its oncogenic activity and regulates stress response. PTPH1 was identified as a substrate of p38γ by unbiased proteomic analysis, and its resultant phosphorylation at Ser-459 occurs in vitro and in vivo through their complex formation. Genetic and pharmacological analyses showed further that Ser-459 phosphorylation is directly regulated by Ras signaling and is important for Ras, p38γ, and PTPH1 oncogenic activity. Moreover, experiments with physiological stimuli revealed a novel stress pathway from p38γ to PTPH1/Ser-459 phosphorylation in regulating cell growth and cell death by a mechanism dependent on cellular environments but independent of canonical MAPK activities. These results thus reveal a new mechanism by which a MAPK regulates Ras oncogenesis and stress response through directly phosphorylating its phosphatase.
Introduction
Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38s, are major signaling cascades that mediate and integrate various signals for a coordinated cellular response such as proliferation, transformation, and cell death (1). The intensity and duration of a MAPK activity are tightly regulated through balanced actions of its activating kinase(s) and of its inactivating phosphatase(s) (2), resulting in changes in target gene expression leading to a given cellular response (3). Although protein phosphatases are known to play a critical role in determining a MAPK activity by positive and negative feedback (4–8), it is not known, however, whether a MAPK can also signal through its phosphatase. Studies of bidirectional signaling cross-talk between a MAPK and its phosphatase may be fundamentally essential to understanding their roles in regulating important biological processes.
p38α and p38γ MAPKs are important p38 family proteins, and previous studies from us as well as others revealed their opposite activity in regulating Ras oncogenesis, stress response, and differentiation (9–11). In response to Ras signaling, p38α phosphorylation is increased, and phosphorylated p38α then functions as a tumor suppressor in vitro (12, 13) and in vivo (14, 15), whereas p38γ expression is increased, and resulting p38γ in turn promotes the oncogenic process independently of (16–18) and dependent on phosphorylation (19, 20). In stress response, although p38γ is similarly activated as pro-apoptotic p38α, it plays an anti-apoptotic role under certain conditions (17, 21–24). Among MAPK family proteins, p38γ is the only member whose expression is induced during differentiation (25), transformation, and/or invasion (16, 18, 19). Moreover, p38γ is overexpressed in primary colon and breast cancer (16, 18) and was recently shown to stimulate breast cancer invasion and/or metastasis (19, 26, 27). These results together indicate that p38γ exerts its dual oncogenic and stress-regulatory activity through phosphorylation and/or increased expression, but the underlying mechanisms remain largely unknown.
p38γ is a unique MAPK with its carboxyl-terminal sequence ETXL serving as a PDZ (PSD-95/Dlg/ZO-1 homology) motif to interact with various PDZ domain-containing proteins (28–30). Through two-hybrid screening we recently identified a protein-tyrosine phosphatase H1 (PTPH1)2 as a p38γ-specific phosphatase through PDZ-mediated interaction and demonstrated its cooperative activity in promoting Ras oncogenesis (18). Moreover, p38γ was shown to increase PTPH1 expression, and levels of p38γ and PTPH1 proteins are co-hyperexpressed in primary colon cancers (18). These results indicate that p38γ may exert its oncogenic activity through regulating PTPH1 expression, but the mechanism for this regulatory event is unclear. Because PDZ binding serves as an important mechanism for protein-protein interaction (31, 32) through which p38γ was previously shown to phosphorylate its substrates (28–30), we tested a hypothesis that it may also allow PTPH1 as a p38γ substrate through which p38γ exerts its dual mitogenic and stress-regulatory activity. Our results showed that p38γ phosphorylates PTPH1 at Ser-459 in vitro and in vivo leading to PTPH1 stabilization, which plays an important role in Ras oncogenesis and stress response. These results revealed a novel mechanism by which a MAPK signals through its phosphatase to regulate cellular response.
EXPERIMENTAL PROCEDURES
Gene Expression/Silencing and Retrovirus and Lentivirus Infection
FLAG-tagged p38γ and p38γ/AGF were stably expressed in IEC-6 cells through G418 selection, and pooled resistant cells were infected with LZRS-K-Ras through puromycin selection (16). To overexpress PTPH1 and its mutants, IEC-6 cells or IEC-6/K-Ras cells were transfected with these plasmids and selected with G418, and pooled cells were used for different analyses. To express p38γ in HCT116 cells, the retroviral vector pLHCX and pLHCX-p38γ were used (18). Tet-inducible expression of MKK6-p38γ fusion protein in MCF-7 cells was described recently (33), whereas Tet-On shRNA against the mutated K-Ras was performed as reported previously (34).
Cell Proliferation, Cell Death, Soft-agar Growth, Colony Formation, and Viability Assays
Cells (5,000) in a 96-well plate were incubated with different reagents for 24 h, and cell proliferation was performed according to the CyQuant NF cell proliferation assay kit manual (Cat: C35006) as described previously (35). To measure cell death, cells were stained with trypan blue, and viable cells were counted and expressed as relative of total cells as described previously(17). For soft-agar growth, 1–1.5 × 104 cells were plated in the growth media containing 0.33% SeaPlaque agarose. Formation of multicellular colonies was visualized and counted 10 or 14 days later. The colonies formed on 60-mm plates were photographed and manually counted, and the relative colony numbers are calculated against respective controls, which typically came from three separate experiments with each in triplicate plates (18). For human colon cancer cells, approximately 200 cells were plated in triplicate in a 6-well plate, and the colonies formed were stained and counted approximately 2 weeks later (35). For the cell proliferation assay, cells were plated at the same density, and viable cells were counted at 24 h and 48 h later after brief staining with trypan blue as described previously (36).
Statistical Analysis
Results from all experiments such as colony formation, soft-agar growth, cell proliferation, viability assays, and some IP/WBs were analyzed by Student's t test, unless specified, for a statistical significant difference.
RESULTS
p38γ Phosphorylates PTPH1 at Ser-459 in Vitro and in Vivo
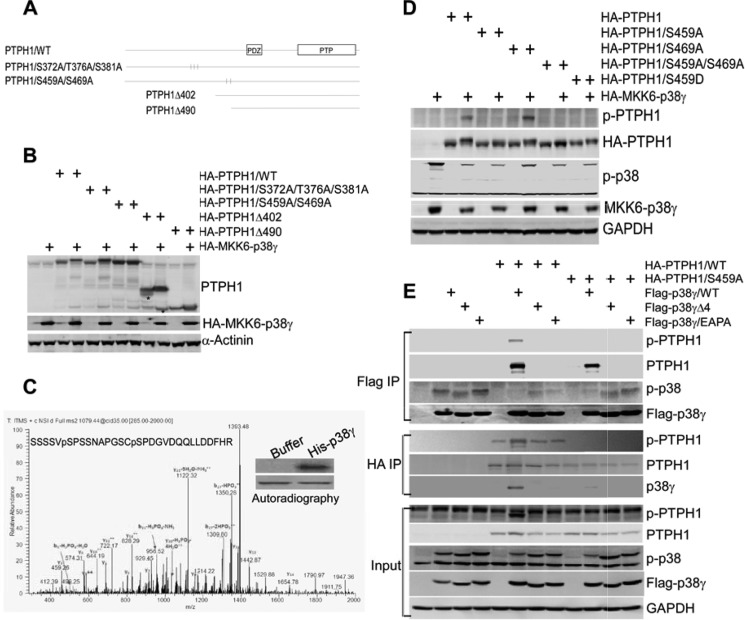
To investigate whether p38γ phosphorylates PTPH1, two deletion mutants were generated by PCR (Fig. 1A), which were co-expressed with and without a constitutively active (CA) p38γ (the MKK6-p38γ fusion construct) (17) in 293T cells, and resulting changes in mobility of expressed proteins were examined by Western blot (WB). In addition, several potential MAPK phosphorylating S/TP motifs (3) were mutated to alanine (A), and the mutated constructs were also included in the analysis. Results in Fig. 1B showed that CA p38γ expression decreased the mobility of PTPH1Δ402 but not PTPH1Δ490, indicating that p38γ may phosphorylate PTPH1 at residues between 402 and 490 (Fig. 1A). The same conclusion is also reached when the in vitro kinase assay was performed by incubating HA-isolated PTPH1 proteins with His-p38γ (supplemental Fig. S1A and supplemental material “Experimental Procedures”). Experiments with the site-specific PTPH1 mutants showed further that the p38γ co-expression reduced the mobility of PTPH1 and its mutant S372A/T376A/S381A without significant effects on the S459A/S469A (Fig. 1, A and B), suggesting that Ser-459 and/or Ser-469 may be the potential phosphorylation sites.
FIGURE 1.
p38γ MAPK phosphorylates PTPH1 at S459. A, molecular structures of PTPH1 and its mutants. B and C, cells (293T) transfected with the indicated constructs, which were then analyzed by WB for mobility changes (B, * a nonspecific band likely from the truncated PTPH1 degradation). The kinase assay was performed by incubation of HA-precipitated PTPH1 with bacterially expressed His-tagged p38γ (12). The resulting mixtures were separated on a SDS-PAGE, and the band corresponding to PTPH1 (C, inset) was excised and digested with trypsin. Phosphorylation sites were mapped by the microcapillary LC/MS/MS with approximately 85% coverage of the PTPH1 amino acid sequence. A phosphopeptide consistent with the phosphorylation at Ser-459 and Ser-469 was identified (C, pS). D, p38γ phosphorylating PTPH1 at Ser-459 but not Ser-469. Cells (293T) were transfected with the indicated plasmid DNAs, which were then analyzed by WB using a specific antibody against the phosphorylated PTPH1/Ser-459. E, PTPH1 phosphorylated at Ser-459 by p38γ. Phosphorylated and unphosphorylated PTPH1/Ser-459 similarly dephosphorylated p-p38γ through PDZ-mediated complex formation. Various plasmids were expressed as indicated, and FLAG/HA precipitates were examined by WB with a portion of whole cell lysates as an input control.
Next, we used the mass spectrometry technique to identify which residues are phosphorylated by p38γ. In this case, HA-tagged PTPH1 was expressed in 293T cells and HA precipitates were then incubated in vitro with His-p38γ in the presence of radioactive isotope. Following a separation by SDS-PAGE, a piece of the gel corresponding to the PTPH1 band was removed, followed by trypsin digestion, and the LC/MS/MS analysis (37) through which a phosphopeptide consistent with the PTPH1 phosphorylation at Ser-459 and Ser-469 was identified (Fig. 1C). Additional analysis showed that the CA p38γ decreased only the mobility of PTPH1 and PTPH1/Ser-469A, but not PTPH1/S459A or PTPH1/S459A/S469A (Fig. 1D), indicating that Ser-459 is likely the residue phosphorylated by p38γ. To demonstrate further and characterize the role of Ser-459 in p38γ-induced PTPH1 phosphorylation, a specific phospho-PTPH1/S459 antibody (p-PTPH1) was generated by PhosphoSolutions®. As can be seen in supplemental Fig. S1B, the antibody recognized GST-PTPH1 but not its S459A mutant after incubation with His-p38γ (top), whereas p38α phosphorylates ATF2 but not PTPH1 in vitro (bottom), indicating its specificity against the phosphorylated PTPH1 at Ser-459. Moreover, the in vivo CA p38γ co-transfection experiment showed that the antibody reacts with the expressed PTPH1 and PTPH1/S469A, but not with the S459A, S459A/S469A, and the phospho-mimic S459D mutant (Fig. 1D), and similar results were also obtained by the FLAG-p38γ co-expression (supplemental Fig. S1C). The alignment analysis showed further that the Ser-459 is conserved in different species (supplemental Fig. S1E), indicating its important role in PTPH1 activity. These results together demonstrated that p38γ specially phosphorylates its phosphatase PTPH1 at Ser-459 in vitro and in vivo, which may play a crucial role in transduction of the MAPK signaling.
p38γ Phosphorylates PTPH1 through PDZ Binding, Which Affects the Phosphatase Activity by a Substrate-dependent Mechanism
We showed previously that PTPH1 decreased p38γ but not p38α phosphorylation in vitro and in vivo (38). An examination of co-expressed p38γ de-phosphorylation by PTPH1 showed that an alteration of Ser-459 to Ala (S459A) or Asp (S459D, a phospho-mimic mutant), did not significantly affect the phosphatase activity (Fig. 1, D and E, and supplemental Fig. S1C), indicating that the PTPH1 phosphorylation by p38γ occurred independent of its catalytic activity. This conclusion is supported further by the fact that p38γ similarly phosphorylated PTPH1 and its catalytic-deficient mutant PTPH1/DA (supplemental Fig. S1F) (39). To show whether Ser-459 phosphorylation affects the phosphatase activity on another substrate, MYC-tagged epidermal growth factor receptor (EGFR) was expressed in 293T cells, Myc precipitates were incubated with GST-PTPH1 in vitro, and the resultant EGFR/Tyr-1173 phosphorylation was examined by a specific antibody. Results in supplemental Fig. S1D showed a dose-dependent decrease in levels of p-EGFR/Tyr-1173 by PTPH1 (top). Of great interest, the co-transfection experiments showed further that expression of PTPH1, but not its S459A mutant, reduced p-EGFR/Tyr-1173 expression (supplemental Fig. S1D, bottom), indicating that the phosphorylation is required for PTPH1 dephosphorylation of EGFR/Tyr-1173. To analyze further the roles of Ser-459 phosphorylation and PTPH1-p38γ binding in PTPH1 catalytic activity, p38γ and its PTPH1-binding deficient mutants were co-expressed with wild-type (WT) and PTPH1/S459A, and expressed proteins were isolated and analyzed for their phosphorylation and binding activities. Because human p38γ binds PTPH1 through its carboxyl-terminal PDZ motif (−ETPL) (18), two separate mutants (p38γΔ4, a truncated mutant with ETPL deleted; p38γ/EAPA, a site-specific mutant with ETPL changed to EAPA) (40) were included in the analysis. Results in Fig. 1E showed that p38γ binds and phosphorylates PTPH1 at Ser-459, whereas both of its mutants fail to show these effects even though their activities (phosphorylations) are increased because of the absence of the phosphatase in the complex, indicating a PTPH1 phosphorylation event dependent on PDZ-mediated complex formation. Analysis of FLAG-p38γ precipitates showed further that PTPH1 and its S459A mutant similarly decreased p38γ phosphorylation (Fig. 1E). These results together indicated that the PTPH1 phosphorylation by p38γ required PDZ-mediated interaction, but the resultant PTPH1 phosphorylation had no effect on its catalytic activity toward p38γ but was required for its dephosphorylation of EGFR. Similar interactions were reported previously for ERK-induced phosphorylation of its phosphatase MAPK phosphatase-1 (4) at Ser-359 and Ser-364 (5). Although the PDZ complex may allow the bidirectional reaction, both WT PTPH1 and its catalytic defective PTPH1/DA are phosphorylated by p38γ at Ser-459, whereas PTPH1 and its S459A mutant have a similar dephosphorylating effect on p-p38γ (Fig. 1, D and E, and supplemental Fig. S1F) (18). Together, these results suggested that PDZ binding may favor PHPH1 phosphorylation instead of p38γ dephosphorylation (Fig. 5E). Thus, p38γ may signal via its phosphatase to exert its biological activity.
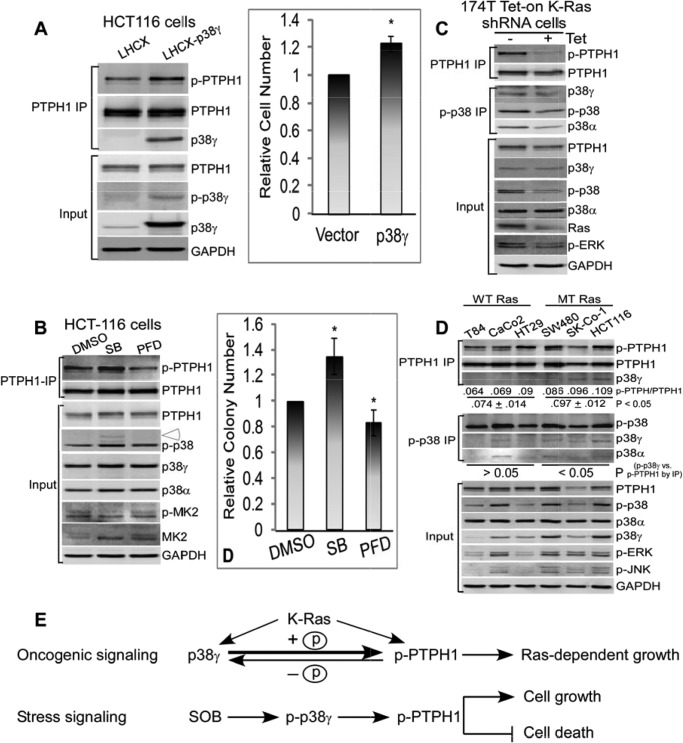
FIGURE 5.
p38γ may promote the K-Ras-dependent colon cancer growth through stimulating PTPH1/Ser-459 phosphorylation. A, K-Ras-mutated HCT116 cells were stably expressed with p38γ by retroviral infection (LHCX-p38γ), and endogenous PTPH1 was isolated and examined for p-PTPH1/S459 expression by WB compared with the vector control (LHCX) (left). The viable cells were counted 48 h after the plating at the same density (right) (36), and results shown (right) are relative cell number over the vector cells (mean ± S.D. (error bars), n = 3, * p < 0.05 versus vector). B, HCT116 cells were incubated with SB (10 μm) or PFD (100 μg/ml) for 24 h, and PTPH1/Ser-459 phosphorylation was examined by IP/WB (left, open arrowhead, p-p38γ), or treated with SB (1 μm) or PFD (20 μg/ml) continuously for 10–14 days for colony formation assay (right) (33). Results of colony formation are from three separate experiments and shown as relative of the solvent control (mean ± S.D.; *, p < 0.05 versus dimethyl sulfoxide). C, Tet-On-inducible siRNA for depleting the mutated K-Ras in 174T human colon cancer cells was performed (± Tet for 72 h) as described (34), and PTPH1/Ser-459 phosphorylation was examined by IP/WB. D, six human colon cancer cell lines were examined for p-PTPH1 expression by PTPH1 IP (top) and for p38γ activity by p-p38 IP (middle). The average level of the phosphorylated PTPH1 over total PTPH1 in PTPH1 precipitates was lower from three WT K-Ras cell lines compared with that from three MT K-Ras cell lines (top), which was further grouped based on the K-Ras status and compared with that of p-p38γ protein expression from total p-p38 precipitates (middle) by the two-tailed Pearson Correlation analysis (middle). E, experimental model shows that in response to the oncogene signaling, p38γ will promote the K-Ras-dependent growth through stimulating PTPH1/Ser-459 phosphorylation (top, indicated by a bold arrow toward p-PTPH) via PDZ-mediated complex formation, whereas in response to the stress signal SOB, p38γ may function as a noncanonical MAPK to specifically stimulate p-PTPH1/S459, which can antagonize the stress-induced growth-inhibition and cell death.
p38γ Signals through Phosphorylating PTPH1/S459 to Increase Ras Transformation
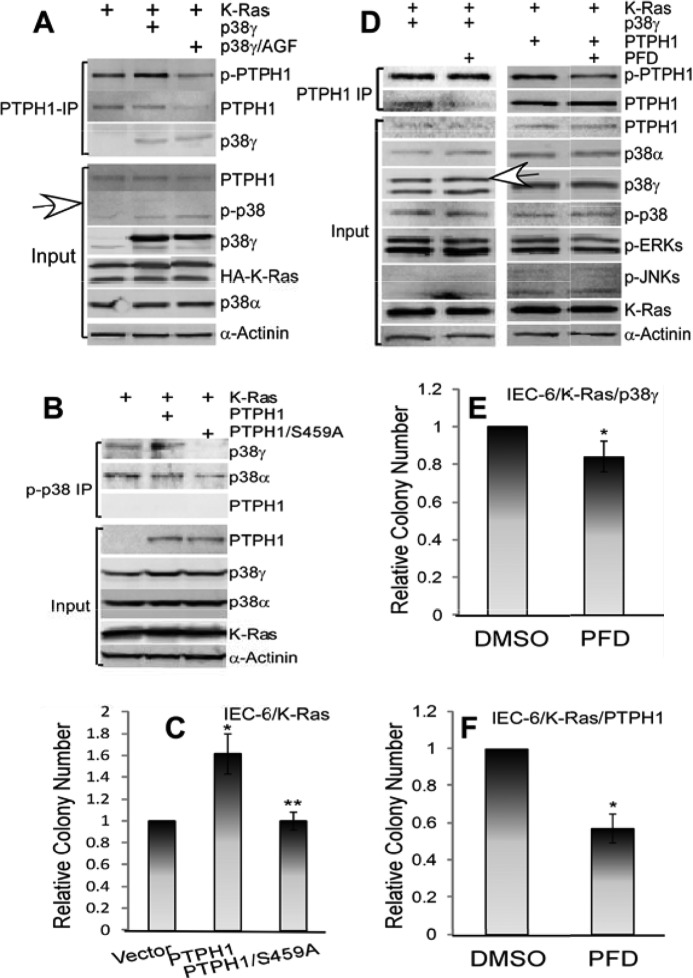
p38γ and PTPH1 were shown previously to be important for Ras transformation in rat intestine epithelial cells (IEC-6) through a PDZ-mediated complex formation (16, 18). To determine the role of p-PTPH1 in p38γ potentiating the Ras transformation, the signaling cross-talk between p38γ and PTPH1 was next dissected in K-Ras transformed IEC-6 cells. Results in Fig. 2A showed that a stable expression of a nonphosphorable p38γ (p38γ/AGF) in IEC-6/K-Ras cells reduced levels of endogenous p-PTPH1 expression, whereas expressing WT p38γ increased these in PTPH1 precipitates compared with the vector-transfected cells. Importantly, increased p-PTPH1 expression by p38γ coupled with an increased soft-agar growth compared with cells expressed with p38γ/AGF, albeit both WT and AGF p38γ increased the colony formation over the vector control, indicated a functional role of Ser-459 phosphorylation in p38γ stimulating Ras transformation (supplemental Fig. S2A). Moreover, knocking down endogenous p38γ by pSUPER-mediated siRNA delivery in these cells decreased both p-PTPH1 expression and colony formation without affecting PTPH1 protein expression (supplemental Fig. S2, B and C) (16, 18). Furthermore, the stable expression of PTPH1/S459A completely abolishes the enhancement of the soft-agar growth compared with cells expressed with the WT protein and with the control vector (Fig. 2, B and C, and supplemental Fig. S2D), pointing further to a critical role of the Ser-459 phosphorylation in the PTPH1 oncogenic activity (18). To demonstrate further the significance of p38γ phosphorylation of PTPH1, IEC-6/K-Ras cells stably expressed with PTPH1 and p38γ were incubated with pirfenidone (PFD), a specific p38γ pharmacological inhibitor (41–43), and PTPH1 precipitates were analyzed by WB. Results in Fig. 2D showed that the incubation of PTPH1-overexpressed cells with PFD decreased significantly the p-PTPH1 expression in PTPH1 precipitates without significant effects on PTPH1 expression in whole cell lysates (input). Importantly, the treatment with PFD inhibited significantly the soft-agar growth of IEC-6/K-Ras cells stably expressed with both p38γ and PTPH1 (Fig. 2, E and F), highlighting further a critical role of the PTPH1 phosphorylation in their oncogenic activity. Whereas p38γ may exert its oncogenic activity through additional yet unknown substrates, the coupling of decreased p-PTPH1 expression with reduced colony growth following the p38γ genetic suppression as well as its pharmacological inhibition (Fig. 2 and supplemental Fig. S2) indicated strongly that p38γ propagated the Ras oncogenic signaling, at least in part, through stimulating directly its phosphatase PTPH1 phosphorylation (Fig. 5E). These results together indicated a critical role of PTPH1/Ser-459 phosphorylation in transduction of its kinase p38γ oncogenic activity.
FIGURE 2.
p38γ promotes the Ras oncogenesis via stimulating PTPH1/Ser-459 phosphorylation. A, coupling of p-PTPH1 expression with the soft-agar growth in IEC-6/K-Ras cells expressed with p38γ and its AGF mutant. IEC-6 cells were stably expressed with p38γ and p38γ/AGF by G418 selection, and resulting cells were then transduced with K-Ras, which were analyzed by PTPH1 IPs/WB (A, open arrowhead, p-p38γ) and by the soft-agar growth (supplemental Fig. S2A). B and C, PTPH1 dependent on Ser-459 for its oncogenic activity. Transformed IEC-6/K-Ras cells (16) were transfected with PTPH1 and PTPH1/S459A through G418 selection, and resistant cells were pooled and analyzed by the soft-agar growth (C) and p-p38 IP/WB (B). Results in C are from three separate experiments with each in triplicate as described in supplemental Fig. S2A and expressed as relative to vector controls (error bars, S.D.; *, p < 0.05; and **, p > 0.05 versus vector, Student's t test). Colonies are from one representative experiment shown in supplemental Fig. S2D. D–F, IEC-6/K-Ras/p38γ and IEC-6/K-Ras/PTPH1 cells incubated with either 50 μg/ml PFD or dimethyl sulfoxide for 24 h for IP/WB (D) or with 50 μg/ml PFD present in the top layer of soft-agar continuously for about 10–14 days for the soft-agar growth (18). E and F, mean of three separate experiments, with each in triplicate, * p < 0.05 versus dimethyl sulfoxide).
PTPH1/Ser-459 Phosphorylation is Increased in Response to Both Mitogenic and Stress Stimuli in Association with p38γ Activation by a Microenvironment-dependent Mechanism and Plays a Role in Increasing Cell Growth and in Inhibiting Cell Death Induced by Sorbitol, but Not by Anisomycin
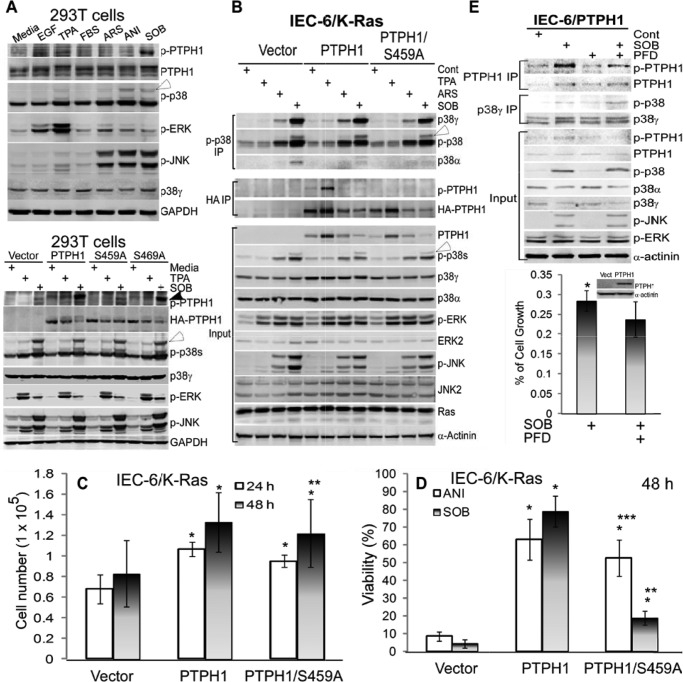
p38γ can be activated by increased phosphorylation and/or expression in response to stress and oncogenic signals (11, 16–18, 33, 43). To demonstrate further the roles of PTPH1/Ser-459 phosphorylation in transduction of p38γ signaling in response to various physiological stimuli, 293T cells were pulse-treated with various reagents (phorbol ester (TBA), epidermal growth factor (EGF), and fetal bovine serum (FBS), and stress (ARS (arsenite), anisomycin (ANI), and sorbitol (SOB)), and the level of p-PTPH1 expression was examined by WB compared with p38γ and other major MAPK phosphorylations. Results in Fig. 3A (top) showed that both mitogenic (TPA and EGF) and stress SOB stimuli induced Ser-459 phosphorylation of endogenous PTPH1, together with increased p-ERKs by EGF/TPA and with elevated p-JNK/p-p38s by ANI and SOB. Of interest, only increased p38γ phosphorylation (indicated by an open arrow) by TPA and SOB, but not by ARS and ANI, correlated with elevated levels of p-PTPH1 expression (Fig. 3A, top), indicating a role of p38γ in PTPH1/Ser-459 phosphorylation by a stimulus-specific mechanism. To dissect further the relationship between p38γ activity and PTPH1 phosphorylation, the role of Ser-459 in cellular response to TPA and SOB was examined further in 293T cells after they were expressed transiently with PTPH1 and its S459A mutant. Results in Fig. 3A (bottom) showed that both endogenous (the vector group) and transfected PTPH1 were phosphorylated at Ser-459 by SOB and TPA in an association with endogenous p38γ (and other p38s) and JNK but not ERK phosphorylation. Because levels of p-p38γ expression showed no significant changes with or without PTPH1 or its mutant expression under these conditions, these results further indicated a role of p38γ in PTPH1 phosphorylation, but not of PTPH1 in p38γ dephosphorylation, a conclusion that was obtained by their co-expression analysis (Fig. 1, D and E, and supplemental Fig. S1C).
FIGURE 3.
PTPH1/Ser-459 phosphorylation is increased in response to both mitogenic and stress stimuli, p38γ regulates stress signaling to stimulate p-PTPH1/Ser-459 expression by a microenvironment-dependent mechanism, and PTPH1 requires Ser-459 to inhibit SOB but not ANI-induced cell death. A, cells (293T) were treated with different reagents for 1 h (EGF at 20 ng/ml, TPA at 200 ng/ml, FBS at 10%, ARS at 100 μm, ANI at 100 μg/ml, and SOB at 0.5 m), which were then analyzed by WB. Bottom panel, PTPH1 and its mutants were transiently expressed in 293T cells for 48 h, followed by treatment with TPA or SOB as above, and effects of expressed PTPH1 and its mutants on p-p38γ and other p-MAPKs expression were examined by WB. Closed and open arrowhead indicate endogenous p-PTPH1 and p-p38γ, respectively. B, IEC-6/K-Ras cells stably expressed with indicated plasmids were treated with stimuli as in A, which were analyzed by IP and WB (open arrowhead, p-p38γ). C, IEC-6/K-Ras cells expressed with indicated plasmids were plated at the same density, and cell growth was determined by counting viable cells at 24 and 48 h (error bars, S.D.; n = 3; *, p < 0.05; and **, p > 0.05 versus vector; recalculated from the control groups of D and supplemental Fig. S3A). D, indicated IEC-6/K-Ras cells were pulse-treated with SOB or ANI as in A, and cell viability was determined 48 h later by the trypan blue exclusion assay (17) (n = 3; *, p < 0.05 versus vector, **, p < 0.05 versus PTPH1, and ***, p > 0.05 versus PTPH1, with the viability of all control groups without ANI/SOB treatment being >95%). Similar results were obtained at 24 h (supplemental Fig. S3A), and associated WB results are shown in supplemental Fig. S3C. E, IEC-6 cells stably expressed with PTPH1 were preincubated with and without PFD for 6 h (50 μg/ml), followed by the pulse exposure to SOB as in A, and resultant effects on p-PTPH1/p-p38γ expression were analyzed by IP/WB (top) and on cell growth by the CyQuent NF cell proliferation kit (35). Results (bottom, n = 3; *, p < 0.05) are expressed as relative to the solvent control, with cells in every group for each of experiments plated in 6 wells with the mean absorbance from 6 wells considered as one experiment.
To demonstrate further the p38γ signaling to its phosphatase (but not vice versa) in an independent cell line, IEC-6/K-Ras cells stably expressed with HA-tagged WT PTPH1 and its S459A mutant (Fig. 2B) were pulse-treated with TPA, ARS, and SOB, followed by IP/WB analysis. Different from the association of increased p-p38γ with elevated p-PTPH1 expression in 293T cells (Fig. 3A), the maximal PTPH1/Ser-459 phosphorylation in HA precipitates correlated with the TPA-induced p-ERK but not with SOB-induced p-p38γ expression in PTPH1-overexpressed cells (Fig. 3B). These effects may be because of different kinetics of p-p38γ and p-PTPH1 in response to TPA and SOB in these two cell lines and/or because of TPA induction and SOB inhibition of the transfected HA-PTPH1 expression in IEC-6/K-Ras cells (Fig. 3, A and B). Because PTPH1 expression and phosphorylation are regulated by mitogenic and stress stimuli, we next investigated their roles in cell growth and cell death. Stable expression of PTPH1 stimulated the growth of IEC-6/K-Ras cells over the vector transfection both at 24 and 48 h, whereas the expressed S459A only significantly increased the cell number at 24 but not at 48 h (Fig. 3C), indicating a role of Ser-459 phosphorylation in sustaining the PTPH1 growth-stimulatory activity. Most significantly, both PTPH1 and PTPH/S459A expressions blocked ANI-induced cell death, whereas the death protective activity was reduced significantly in PTPH1/S459A-expressed cells in response to SOB (Fig. 3D and supplemental Fig. S3A), indicating a specific role of Ser-459 phosphorylation in PTPH1 protection against SOB, but not ANI-induced cell death. Similarly, there was an attenuated growth inhibition by both ANI and SOB in PTPH1 and PTPH1/S459A-expressed IEC-6/K-Ras cells compared with the control cells, which, however, was lost only in the mutant PTPH1-expressed cells in response to SOB but not ANI (supplemental Fig. S3B). These results, together with stimulation of p38γ phosphorylation and/or expression and with stimulation of p-PTPH1/Ser-459 expression by SOB but not ANI (Fig. 3, A and B, and supplemental Fig. S3C), indicated a specific pathway from p38γ to PTPH1 through Ser-459 phosphorylation in response to the osmotic stress SOB. Thus, PTPH1 phosphorylation at Ser-459 played an important role both in promoting cell growth and in suppressing SOB-induced cell death/growth inhibition in Ras-transformed IEC-6 cells.
The dissociation of SOB stimulating p-p38γ expression from increasing p-PTPH1 expression in IEC-6/K-Ras cells may be because in the transformed cells there is the oncogenic Ras signaling that would undermine the stress signal transduction and/or there is a lack of a sufficient amount of PTPH1 protein expression. To test these possibilities, normal IEC-6 cells were stably transfected with PTPH1 and were then pulse-exposed to SOB, followed by PTPH1/p38γ IP and WB analyses. Results in Fig. 3E show that levels of p-p38γ and p-PTPH1 were both increased by the SOB treatment in the absence of the ERK stimulation but together with the increased p-JNK/p-p38s expression, indicating that p38γ may signal stimulation of its phosphatase phosphorylation dependent on cellular environments. Importantly, a 6-h preincubation with PFD substantially decreased levels of p-PTPH1 expression in PTPH1 precipitates and in whole cell lysates. It is worth noting that under this experimental condition, the SOB treatment led to >70% of growth inhibition, which was significantly enhanced by PFD (Fig. 3E, bottom), suggesting that the resultant p38γ activity may be proliferative to antagonize the stress-induced growth inhibition likely through phosphorylating PTPH1/Ser-459. The kinetic analysis of PTPH1/p-p38 precipitates showed further that the SOB-induced peak p-p38γ in IEC-6 cells and the TPA-induced maximal p-p38 γ expression in IEC-6/K-Ras cells occurred earlier than the maximal p-PTPH1 expression (supplemental Fig. S3, D and E), thus strengthening further the role of p38γ in stimulating p-PTPH1/Ser-459 expression. These results together demonstrated that p38γ can mediate both mitogenic and stress signaling to stimulate PTPH1/Ser-459 phosphorylation by a mechanism depending on cellular environments. Results with the pharmacological inhibitor PFD (Figs. 2D and 3E) and from PTPH1 and its S459A expressed cells (Fig. 3, C and D, and supplemental Fig. S3, A–C) indicated further that p38γ may be a specific kinase to regulate SOB-induced PTPH1/Ser-459 phosphorylation that is important for PTPH1 to antagonize the stress-induced growth-inhibition and cell death.
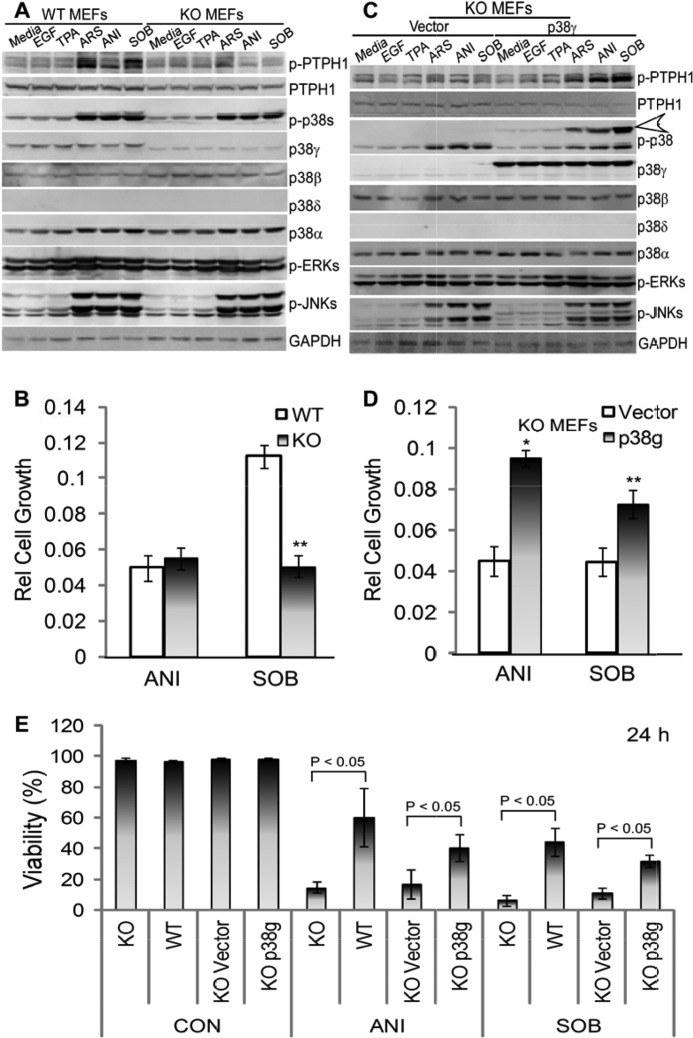
p38γ Is Necessary and Sufficient for Stress-induced p-PTPH1 Expression in Regulating the Growth Inhibition and Cell Death in Mouse Embryonic Fibroblasts
To demonstrate further the role of p38γ in the PTPH1 phosphorylation by physiological stimuli, Ras-immortalized mouse embryonic fibroblasts with (p38γ−/−, KO) and without p38γ knock-out (p38γ+/+, WT) (20, 30) were incubated with these reagents, and the resultant effects on p-PTPH1 expression were analyzed by direct Western blotting. Results in Fig. 4A showed that unlike 293T and IEC-6 cells, levels of the p-PTPH1 expression were increased significantly by treatment with all stress stimuli (ARS, ANI, and SOB), whereas only its minimal activation by mitogenic agents EGF and TPA was observed. Of great interest, the resultant p-PTPH1 elevation by the stress stimuli was either completely (ANI and SOB) or greatly (ARS) abolished in p38γ KO cells where there are no detectable changes in p-p38/p-JNK expression (Fig. 4A), indicating further a specific stress pathway through p38γ phosphorylating its substrate PTPH1/Ser-459 independent of other major MAPK activity. Although incubation with SOB led to a significant decrease in both cell growth and cell death in p38γ KO cells compared with WT cells, the ANI treatment only resulted in elevated cell death but not reduced growth in p38γ-null cells (Fig. 4, B and E, and supplemental Fig. S4, A and C), likely as a result of different sensitivities of these assays. However, both SOB and ANI incubation caused a more significant growth inhibition in p38γ KO cells (at least for the 24-h treatment) (supplemental Fig. S4D), thus pointing to a general antagonistic activity of p38γ against stress-induced growth inhibition. Because there are no differences in cell viability (Fig. 4E and supplemental Fig. S4C) and cell growth (data not shown) in p38γ WT and KO cells without stress treatment, these results indicated that endogenous p38γ is not proliferative but is inhibitory to SOB/ANI-induced cell death in mouse fibroblast cells.
FIGURE 4.
p38γ is necessary and sufficient for stress-induced p-PTPH1/Ser-459 expression independent of major MAPK activities and is critical for stress-induced growth-inhibition and cell death. A–D, mouse embryonic cells (MEFs) with (KO) and without (WT) p38γ knock-out that were immortalized by H-Ras (20) (A and B) and (for KO cells that were stably reexpressed further with p38γ or vector (C and D)) were treated with indicated agents for 1 h (A and C) or 24 h (B and D) (EGF, 20 ng/ml; TPA, 200 ng/ml; ANI, 100 μg/ml; ARS, 100 μm; and SOB, 0.5 m). Protein expression and phosphorylation were analyzed by WB (A and C, open arrowhead, p-p38γ), and cell proliferation was measured with the CyQuent NF cell proliferation kit (B and D) as described (35). The data in B and D are relative to solvent control from one representative experiment (mean of 6 wells, error bars, S.D.; **, p < 0.05 versus WT cells in B; *, ** p < 0.05 versus vector-transfected KO MEFs in D) and similar results were obtained from an independent experiment (supplemental Fig. S4, A and B). E, indicated cells were cultured with ANI or SOB for 24 h as in B and D and cell viability (n = 3) was determined as in Fig. 3D. Similar results were obtained at 48 h (supplemental Fig. S4C).
To demonstrate further the role of p38γ signaling through p-PTPH1/S459 in stress response, the p38γ KO cells were reexpressed with p38γ by retroviral infection, and the resultant effect on PTPH1 phosphorylation and cell growth/death was assessed. Results in Fig. 4C show that the p38γ rescue restored the p-PTPH1 stimulation by all stress stimuli without significant impacts on p-ERK, p-JNKs, and p-p38α/β expression compared with the vector-expressed cells, thus demonstrating further a necessary and sufficient role of p38γ MAPK in stress-stimulating p-PTPH1/459 expression independent of classical MAPK pathways. Importantly, the p38γ reexpression significantly attenuates the growth inhibition and reduces substantially the cell death in response to both SOB and ANI (Fig. 4, D and E, and supplemental Fig. S4, B–D). Together, these results demonstrated a specific-stress p38γ pathway to stimulate its phosphatase phosphorylation at Ser-459 independent of major MAPKs in antagonizing stress-induced cell death/growth-inhibition in mouse fibroblasts.
p38γ Phosphorylating PTPH1/Ser-459 Plays a Crucial Role in K-Ras-dependent Human Colon Cancer Growth
Depleting endogenous p38γ and/or PTPH1 was shown previously to inhibit the K-Ras-mutated colon cancer growth in vitro and/or in nude mice (16, 18). Because experiments in IEC-6/K-Ras cells have revealed a crucial role of Ser-459 in p38γ stimulating Ras transformation (Fig. 2), we wished next to determine whether p38γ directly dictates endogenous PTPH1/Ser-459 phosphorylation thereby promoting the K-Ras-dependent growth in human colon cancer cells. Examining PTPH1 precipitates from the p38γ-forced expressed and K-Ras-mutated HCT116 human colon cancer cells showed that the p38γ expression increased levels of p-PTPH1 expression in association with an increased cell growth, indicating that p38γ may increase the K-Ras-dependent growth through increasing p-PTPH1 expression (Fig. 5A). The stimulation of endogenous p-PTPH1/S459 expression can be extended to an overexpression of the p38 activator MKK6 (13) through an adenoviral infection in IEC-6 cells (supplemental Fig. S5A, left). Furthermore, Tet-inducible expression (Tet-On) of the CA p38γ in MCF-7 breast cancer cells (33) also increased levels of p-PTPH1 expression (supplemental Fig. S5A, right). These results together indicated that p38γ and/or its activator can stimulate the endogenous PTPH1 phosphorylation in different cell lines.
The significance of the p38γ signaling to PTPH1 was examined further by application of p38 pharmacological inhibitors. Although SB203598 (SB) is an established p38α/β inhibitor (44), it also activates the cellular Raf (45). Our recent studies showed further that SB increased cellular level of p38γ protein expression through its p38α antagonistic activity (17, 20). Therefore, in addition to the p38γ inhibitor PFD, SB was also included in our analysis to assess the role of endogenous p38γ activity in regulating the PTPH1 phosphorylation and the K-Ras-dependent growth. As expected, results in Fig. 5B showed that the p38γ inhibition by PFD reduced p-PTPH1 expression and the colony formation as observed in IEC-6/K-Ras cells (Fig. 2, D–F), whereas an opposite effect on both was observed with SB. These correlative analyses together indicated that p38γ may partially execute its oncogenic activity in K-Ras-mutated colon cancer cells through stimulating p-PTPH1/Ser-459 expression.
To examine whether the mutated K-Ras directly controls the p38γ/PTPH1 axis, we used a Tet-On system to deliver the specific shRNAs to deplete the mutated (MT) but not wild-type (WT) K-Ras (34). Results in Fig. 5C showed that silencing the MT K-Ras in human 174T human colon cancer cells decreased levels of both p-p38γ and p-PTPH1/Ser-459 protein expression. Because the K-Ras knockdown in this system was shown previously to decrease the malignant growth in vitro and in mice (34), these results indicated that the MT K-Ras may also promote the malignant growth through stimulating the p-p38γ/p-PTPH pathway. Moreover, analysis of PTPH1/p-p38 precipitates from a group of human colon cancer cell lines showed that levels of p-PTPH1 protein expression are increased significantly in MT K-Ras cells over those expressing WT K-Ras, which was coupled further with an increased p-p38γ activity in cells harboring MT, but not WT, K-Ras (Fig. 5D). These results, together with those obtained through pharmacological and genetic approaches (Fig. 5, A and B), demonstrated further a critical role of the p38γ activity in promoting the K-Ras-dependent malignant growth through stimulating PTPH1/Ser-459 phosphorylation.
The p38γ-forced expression in normal IEC-6 cells was shown previously to increase PTPH1 expression (18), and we wished next to determine whether p38γ increases PTPH1 protein stability through stimulating Ser-459 phosphorylation. Because p38γ requires the PTPH1-binding activity to phosphorylate PTPH1/Ser-459 (Fig. 1E), 293T cells were transiently expressed with p38γ and its PTPH1-binding deficient mutant, and the endogenous PTPH1 protein stability was examined by WB after incubation with a protein synthesis inhibitor cycloheximide. Indeed, the p38γ expression appeared to attenuate the PTPH1 degradation over the vector expression, whereas an opposite effect was observed when the mutant p38γ was expressed (18) (supplemental Fig. S5B), indicating that p38γ may stabilize PTPH1 through PDZ binding. To show whether this effect is executed through Ser-459, PTPH1, PTPH1/S459A, and PTPH1/S459D mutants were expressed transiently in 293T cells, and expressed PTPH1 proteins were examined by Western blotting after cycloheximide. Results in supplemental Fig. S5C showed that the phosphatase appeared to be degraded less in the “D” form but more in the “A” form compared with the WT protein, suggesting that the Ser-459 was both necessary and sufficient in maintaining the PTPH1 stability. Experiments in HCT116 cells showed further that whereas the p38γ expression and depletion alone had no substantial effect on PTPH1 expression, the p38γ knockdown appeared to increase PTPH1 degradation (Fig. 5A and supplemental Fig. S5D). A reduced MKP-1 degradation was reported previously after ERK2-induced phosphorylation (5). Therefore, p38γ may maintain its phosphatase PTPH1 stability through stimulating Ser-459 phosphorylation as observed for the ERK2/MKP-1 interaction, and the resulting PTPH1 abundance may increase further the transduction of the kinase signaling to promote the Ras-dependent growth and/or to suppress stress-induced growth-inhibition/cell death (Fig. 5E).
DISCUSSION
MAPKs function through coordinative phosphorylation and dephosphorylation events to regulate dynamic cellular programs leading to various biological responses. Although multiple levels of signaling cross-talks between MAPKs and their phosphatases have been reported (46), the biological outcome of these signaling interactions mostly has not been demonstrated because of their transient and bidirectional nature. Our previous studies have demonstrated a PDZ-mediated oncogenic complex of p38γ MAPK with its phosphatase PTPH1 in promoting Ras oncogenesis, but the mechanism involved remains unknown (18). Here, we showed that p38γ may increase Ras transformation and Ras-dependent colon cancer growth through directly phosphorylating PTPH1/Ser-459. Moreover, p38γ was shown to specifically mediate stress signaling conferred by SOB to stimulate p-PTPH1/Ser-459 expression, which functioned to antagonize the SOB-induced growth-inhibition and/or cell death independent of other major MAPK pathways. These results together revealed a new avenue by which a MAPK signals by utilizing its phosphatase as a substrate to regulate Ras oncogenesis and stress response.
Of a great interest, our results showed that PTPH1 phosphorylation by p38γ depended on their binding but was independent of phosphatase activity. Because p38γ depended on its phosphorylation to interact with PTPH1 (18) and PTPH1 bound p38γ independent of catalytic activity and independent of Ser-459 phosphorylation (Fig. 1E and supplemental Fig. S1F), these results suggested that the PDZ complex may function mostly to facilitate the PTPH1 phosphorylation, albeit their initial binding may dephosphorylate p38γ leading to its dissociation from the complex (Fig. 5E). The loss of the phosphatase catalytic activity by PTPH1/S459A toward EGFR, but not p38γ (Fig. 1, D and E, and supplemental Fig. S1, C and D) highlighted further the signaling fidelity from the kinase to its phosphatase, and not vice versa, albeit the mutant may compromise the PTPH1 catalytic activity to other substrate(s). These results, together with stimulating PTPH1 expression by Ras and p38γ (18) and with increasing PTPH1 stability by p38γ dependent on their binding and dependent on Ser-459 (supplemental Fig. S5), indicated a cellular functional unit of the PDZ-mediated kinase/phosphatase complex in maintaining p-PTPH1 expression. Most critically, this complex was shown to be important for Ras-dependent malignant growth through stimulating the PTPH1 phosphorylation as demonstrated with a pharmacological p38γ inhibitor PFD and/or p38α/β inhibitor SB, the p38γ-specific siRNA, and/or their nonphosphorable mutants (Figs. 2, 3, 5, and supplemental Figs. S2 and S3). Whereas previous studies have established a regulatory role of ERK and p38 MAPKs (12, 15, 47) in the Ras oncogene activity, our results may be the first showing that a MAPK promoted Ras oncogenesis through phosphorylating its phosphatase. Regulation of p38γ activity by PFD and/or SB may have therapeutic potentials to control Ras-dependent malignancies.
Our demonstration of p38γ-mediated PTPH1/Ser-459 phosphorylation in antagonizing SOB-induced growth inhibition and cell death independent of JNK/p38α activities (Fig. 3 and supplemental Fig. S3) is another important discovery. Both JNK/p38 MAPKs (48) and protein-tyrosine phosphatases (49) are known to play a critical role in regulating stress response. However, because of multiple cellular events for a given stress stimulus that may occur by a cell type and/or stimulus-specific or even by a time- and/or concentration-dependent mechanism, whether there exists a specific pathway for a certain chemical still remains controversial. Moreover, mapping stress pathways is complicated further by redundant functions of JNK and p38 MAPKs. TAB1, a subunit of the kinase TAK1, for example, was shown previously to be phosphorylated by p38α at several serine residues in response to SOB and other stress stimuli, but whether these stress stimuli stimulate the TAB1 phosphorylation through p38s and/or JNKs remains unknown (50). MKP3, on the other hand, can inactivate both ERK2 and p38α in vitro and in vivo (51), but the cellular biological consequence of this interaction remains unknown (52). Therefore, whether there is a functional and specific MAPK/phosphatase interaction inside cells that specifically regulates stress-induced cell death and/or a growth inhibition to a given stimulus has never been demonstrated. Our studies presented here, on the other hand, demonstrated that p38γ through PDZ binding is specifically required for the stress signal SOB (but not ARS and ANI) to stimulate PTPH1/Ser-459 phosphorylation, and resultant p-PTPH1/Ser-459 in turn specifically functions to reduce the SOB-induced growth inhibition and cell death in IEC-6 cells independent of JNK/p38α activities (Fig. 3 and supplemental Fig. S3). These results are similar to our recent observation that p38γ is specifically activated by DNA topoisomerase IIα inhibitors in breast cancer, and resultant p38γ phosphorylates topoisomerase IIα at Ser-1524 leading to the stabilization of topoisomerase IIα protein (33). Thus, p38γ may be a noncanonical MAPK that functions to mediate stress signaling to cellular targets via phosphorylation and stabilization by a stimulus-specific mechanism.
Supplementary Material
Acknowledgments
We thank Drs. N. K. Tonks, J. Han, A. Cuenda, C. B. Gambacorti-Passerini, M. C. Hung, and M. Dwindle for providing various reagents; Drs. Q. Xiang and A. Szabo for statistical analysis; and Drs. K. Stone and S. Hildonen for mass spectrometry analysis.
This study was supported, in whole or in part, by National Institutes of Health Grant 2R01 CA91576. This work was also supported by a Department of Veterans Affairs Merit Review and the Cancer Center of Medical College of Wisconsin (Breast Cancer Show-House).

This article contains supplemental “Experimental Procedures” and supplemental Figs. S1–S5.
- PTPH1
- protein-tyrosine phosphatase H1
- IP
- immunoprecipitation
- WB
- Western blot
- CA
- constitutively active
- p-PTPH1
- phospho-PTPH1
- EGFR
- epidermal growth factor receptor
- PFD
- pirfenidone
- TPA
- 12-O-tetradecanoylphorbol-13-acetate
- ARS
- arsenite
- ANI
- anisomycin
- SOB
- sorbitol
- SB
- SB203598
- MT
- mutated.
REFERENCES
- 1. Raman M., Chen W., Cobb M. H. (2007) Differential regulation and properties of MAPKs. Oncogene 26, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 2. Barr A. J., Knapp S. (2006) MAPK-specific tyrosine phosphatases: new targets for drug discovery? Trends Pharmacol. Sci. 27, 525–530 [DOI] [PubMed] [Google Scholar]
- 3. Avruch J. (2007) MAP kinase pathways: the first 20 years. Biochim. Biophys. Acta 1773, 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun H., Charles C. H., Lau L. F., Tonks N. K. (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75, 487–493 [DOI] [PubMed] [Google Scholar]
- 5. Brondello J. M., Pouysségur J., McKenzie F. R. (1999) Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286, 2514–2517 [DOI] [PubMed] [Google Scholar]
- 6. Camps M., Nichols A., Gillieron C., Antonsson B., Muda M., Chabert C., Boschert U., Arkinstall S. (1998) Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280, 1262–1265 [DOI] [PubMed] [Google Scholar]
- 7. Lin Y. W., Chuang S. M., Yang J. L. (2003) ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J. Biol. Chem. 278, 21534–21541 [DOI] [PubMed] [Google Scholar]
- 8. Lin Y. W., Yang J. L. (2006) Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 281, 915–926 [DOI] [PubMed] [Google Scholar]
- 9. Lassar A. B. (2009) The p38 MAPK family, a pushmi-pullyu of skeletal muscle differentiation. J. Cell Biol. 187, 941–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loesch M., Chen G. (2008) The p38 MAPK stress pathway as a tumor suppressor or more? Front. Biosci. 13, 3581–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hou S., Lepp A., Chen G. (2010) p38γ MAP kinase. UCSD Nat. Mol. Pages, doi: 10.1038/mp.a001720.01 [DOI] [Google Scholar]
- 12. Chen G., Hitomi M., Han J., Stacey D. W. (2000) The p38 pathway provides negative feedback for Ras proliferative signaling. J. Biol. Chem. 275, 38973–38980 [DOI] [PubMed] [Google Scholar]
- 13. Qi X., Tang J., Pramanik R., Schultz R. M., Shirasawa S., Sasazuki T., Han J., Chen G. (2004) p38 MAPK activation selectively induces cell death in K-Ras-mutated human colon cancer cells through regulation of vitamin D receptor. J. Biol. Chem. 279, 22138–22144 [DOI] [PubMed] [Google Scholar]
- 14. Bulavin D. V., Phillips C., Nannenga B., Timofeev O., Donehower L. A., Anderson C. W., Appella E., Fornace A. J., Jr. (2004) Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16Ink4a-p19Arf pathway. Nat. Genet. 36, 343–350 [DOI] [PubMed] [Google Scholar]
- 15. Dolado I., Swat A., Ajenjo N., De Vita G., Cuadrado A., Nebreda A. R. (2007) p38α MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 11, 191–205 [DOI] [PubMed] [Google Scholar]
- 16. Tang J., Qi X., Mercola D., Han J., Chen G. (2005) Essential role of p38γ in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 280, 23910–23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qi X., Pohl N. M., Loesch M., Hou S., Li R., Qin J. Z., Cuenda A., Chen G. (2007) p38α antagonizes p38γ activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J. Biol. Chem. 282, 31398–31408 [DOI] [PubMed] [Google Scholar]
- 18. Hou S. W., Zhi H. Y., Pohl N., Loesch M., Qi X. M., Li R. S., Basir Z., Chen G. (2010) PTPH1 dephosphorylates and cooperates with p38γ MAPK to increase Ras oncogenesis through PDZ-mediated interaction. Cancer Res. 70, 2901–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qi X., Tang J., Loesch M., Pohl N., Alkan S., Chen G. (2006) p38γ mitogen-activated protein kinase integrates signaling cross-talk between Ras and estrogen receptor to increase breast cancer invasion. Cancer Res. 66, 7540–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loesch M., Zhi H. Y., Hou S. W., Qi X. M., Li R. S., Basir Z., Iftner T., Cuenda A., Chen G. (2010) p38γ MAPK cooperates with c-Jun in trans-activating matrix metalloproteinase 9. J. Biol. Chem. 285, 15149–15158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuenda A., Alonso G., Morrice N., Jones M., Meier R., Cohen P., Nebreda A. R. (1996) Purification and cDNA cloning of SAPKK3, the major activator of RK/p38 in stress- and cytokine-stimulated monocytes and epithelial cells. EMBO J. 16, 4156–4164 [PMC free article] [PubMed] [Google Scholar]
- 22. Cuenda A., Cohen P., Buée-Scherrer V., Goedert M. (1997) Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6): comparison of the specificities of SAPK3 and SAPK2 (RK/p38). EMBO J. 16, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu C. C., Wu X., Han J., Sun P. (2010) p38γ regulates UV-induced checkpoint signaling and repair of UV-induced DNA damage. Protein Cell 1, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kukkonen-Macchi A., Sicora O., Kaczynska K., Oetken-Lindholm C., Pouwels J., Laine L., Kallio M. J. (2011) Loss of p38γ MAPK induces pleiotropic mitotic defects and massive cell death. J. Cell Sci. 124, 216–227 [DOI] [PubMed] [Google Scholar]
- 25. Cuenda A., Cohen P. (1999) Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274, 4341–4346 [DOI] [PubMed] [Google Scholar]
- 26. Zhang H., Meng F., Liu G., Zhang B., Zhu J., Wu F., Ethier S. P., Miller F., Wu G. (2011) Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 71, 1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng F., Zhang H., Liu G., Kreike B., Chen W., Sethi S., Miller F. R., Wu G. (2011) p38γ mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly(ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia 13, 472–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasegawa M., Cuenda A., Spillantini M. G., Thomas G. M., Buée-Scherrer V., Cohen P., Goedert M. (1999) Stress-activated protein kinase-3 interacts with the PDZ domain of α1-syntrophin: a mechanism for specific substrate recognition. J. Biol. Chem. 274, 12626–12631 [DOI] [PubMed] [Google Scholar]
- 29. Sabio G., Reuver S., Feijoo C., Hasegawa M., Thomas G. M., Centeno F., Kuhlendahl S., Leal-Ortiz S., Goedert M., Garner C., Cuenda A. (2004) Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38γ and ERK1/ERK2. Biochem. J. 380, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabio G., Arthur J. S., Kuma Y., Peggie M., Carr J., Murray-Tait V., Centeno F., Goedert M., Morrice N. A., Cuenda A. (2005) p38γ regulates the localization of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moscat J., Diaz-Meco M. T., Albert A., Campuzano S. (2006) Cell signaling and function organized by PB1 domain interactions. Mol. Cell 23, 631–640 [DOI] [PubMed] [Google Scholar]
- 32. Smock R. G., Gierasch L. M. (2009) Sending signals dynamically. Science 324, 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qi X., Hou S., Lepp A., Li R., Basir Z., Lou Z., Chen G. (2011) Phosphorylation and stabilization of topoisomerase IIα protein by p38γ mitogen-activated protein kinase sensitize breast cancer cells to its poisons. J. Biol. Chem. 286, 35883–35890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mologni L., Dekhil H., Ceccon M., Purgante S., Lan C., Cleris L., Magistroni V., Formelli F., Gambacorti-Passerini C. B. (2010) Colorectal tumors are effectively eradicated by combined inhibition of β-catenin, K-Ras, and the oncogenic transcription factor ITF2. Cancer Res. 70, 7253–7263 [DOI] [PubMed] [Google Scholar]
- 35. Zhi H. Y., Hou S. W., Li R. S., Basir Z., Xiang Q., Szabo A., Chen G. (2011) PTPH1 cooperates with vitamin D receptor to stimulate breast cancer growth through their mutual stabilization. Oncogene 30, 1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qi X., Borowicz S., Pramanik R., Schultz R. M., Han J., Chen G. (2004) Estrogen receptor inhibits c-Jun-dependent stress-induced cell death by binding and modifying c-Jun activity in human breast cancer cells. J. Biol. Chem. 279, 6769–6777 [DOI] [PubMed] [Google Scholar]
- 37. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hou Z., Peng H., White D. E., Wang P., Lieberman P. M., Halazonetis T., Rauscher F. J., 3rd. (2010) 14-3-3 binding sites in the snail protein are essential for snail-mediated transcriptional repression and epithelial-mesenchymal differentiation. Cancer Res. 70, 4385–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang S. H., Liu J., Kobayashi R., Tonks N. K. (1999) Identification of the cell cycle regulator VCP (p97/CDC48) as a substrate of the band 4.1-related protein-tyrosine phosphatase PTPH1. J. Biol. Chem. 274, 17806–17812 [DOI] [PubMed] [Google Scholar]
- 40. He J., Bellini M., Xu J., Castleberry A. M., Hall R. A. (2004) Interaction with cystic fibrosis transmembrane conductance regulator-associated ligand (CAL) inhibits β1-adrenergic receptor surface expression. J. Biol. Chem. 279, 50190–50196 [DOI] [PubMed] [Google Scholar]
- 41. Ozes O., Blatt L. M., Seiwert S. D. (August 5, 2008) U. S. Patent 7,407,973 B2, 1–46
- 42. Moran N. (2011) p38 kinase inhibitor approved for idiopathic pulmonary fibrosis. Nat. Biotechnol. 29, 301. [DOI] [PubMed] [Google Scholar]
- 43. Qi X., Zhi H., Lepp A., Wang P., Huang J., Basir Z., Chitambar C. R., Myers C. R., Chen G. (2012) p38γ mitogen-activated protein kinase (MAPK) confers breast cancer hormone sensitivity by switching estrogen receptor (ER) signaling from classical to nonclassical pathway via stimulating ER phosphorylation and c-Jun transcription. J. Biol. Chem. 287, 14681–14691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McLaughlin M. M., Kumar S., McDonnell P. C., Van Horn S., Lee J. C., Livi G. P., Young P. R. (1996) Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J. Biol. Chem. 271, 8488–8492 [DOI] [PubMed] [Google Scholar]
- 45. Kalmes A., Deou J., Clowes A. W., Daum G. (1999) Raf-1 is activated by the p38 mitogen-activated protein kinase inhibitor, SB203580. FEBS Lett. 444, 71–74 [DOI] [PubMed] [Google Scholar]
- 46. Owens D. M., Keyse S. M. (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26, 3203–3213 [DOI] [PubMed] [Google Scholar]
- 47. Shin S., Dimitri C. A., Yoon S. O., Dowdle W., Blenis J. (2010) ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol. Cell 38, 114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wagner E. F., Nebreda A. R. (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 [DOI] [PubMed] [Google Scholar]
- 49. Tonks N. K. (2005) Redox redux: revisiting PTPs and the control of cell signaling. Cell 121, 667–670 [DOI] [PubMed] [Google Scholar]
- 50. Cheung P. C., Campbell D. G., Nebreda A. R., Cohen P. (2003) Feedback control of the protein kinase TAK1 by SAPK2a/p38α. EMBO J. 22, 5793–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y. Y., Wu J. W., Wang Z. X. (2011) Mitogen-activated protein kinase (MAPK) phosphatase 3-mediated cross-talk between MAPKs ERK2 and p38α. J. Biol. Chem. 286, 16150–16162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jurek A., Amagasaki K., Gembarska A. (2009) Negative and positive regulation of MAPK phosphatase 3 controls platelet-derived growth factor-induced ERK activation. J. Biol. Chem. 284, 4626–4634 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.