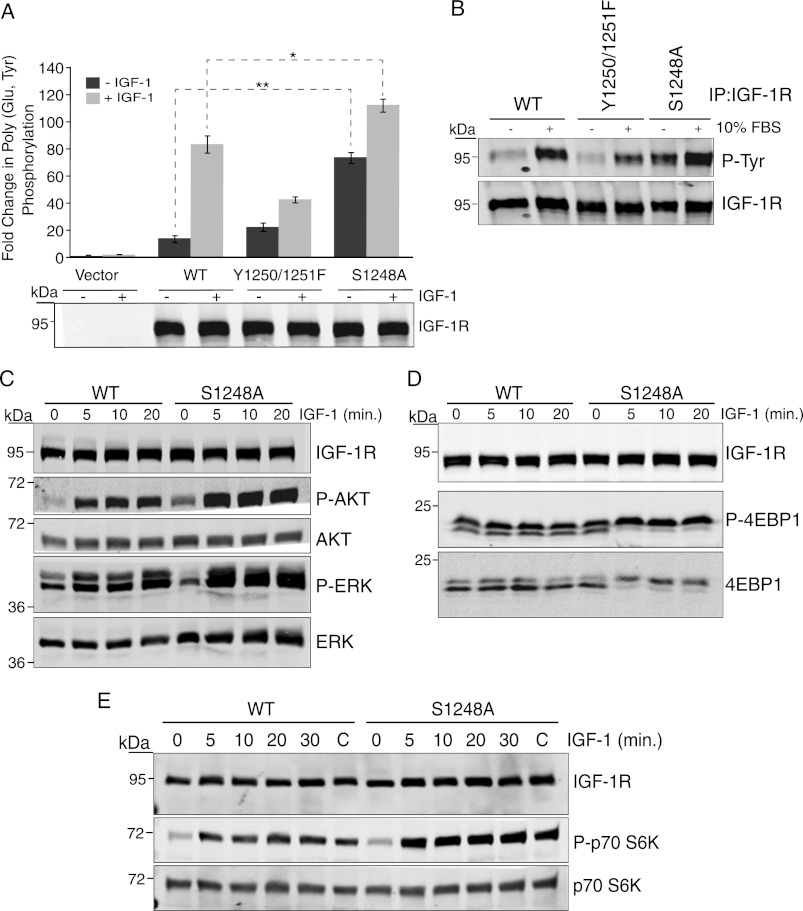
FIGURE 1.
Mutation of Ser-1248 increases IGF-1R tyrosine kinase activity and signaling. A, clones of R− cells stably expressing pcDNA3 empty vector (Vector), pcDNA3 IGF-1R WT, or IGF-1R mutants Tyr-1250/Tyr-1251 and S1248A (R−/IGF-1R WT, R−/IGF-1R Y1250F/Y1251F, and R−/IGF-1R S1248A) were serum-starved for 4 h (−) or stimulated with IGF-1 for 15 min (+). Immunoprecipitated (IP) IGF-1R was assessed for in vitro kinase activity toward poly(Glu,Tyr) in the presence of [γ-32P]ATP. Data from three independent experiments are presented as the fold change in kinase activity of IGF-1R immunoprecipitates relative to control (immunoprecipitates from empty vector cells). Error bars reflect the standard deviation, **, p < 0.005, and *, p < 0.01, calculated by Student's t test. Levels of IGF-1R determined by immunoblotting with anti-IGF-1R antibody are indicated. B, R−/IGF-1R WT, R−/IGF-1R Y1250F/Y1251F, and R−/IGF-1R S1248A cells were serum-starved for 4 h or maintained in serum. IGF-1R was immunoprecipitated and then immunoblotted for anti-phosphotyrosine content. Blots were stripped and re-probed with anti-IGF-1R antibody. C, R−/IGF-1R WT, R−/IGF-1R Y1250F/Y1251F, and R−/IGF-1R S1248A cells were serum-starved for 4 h and then stimulated with IGF-1 for the indicated times. Cell lysates were immunoblotted with anti-IGF-1R, -phospho-Akt, -Akt, -phospho-ERK, and -ERK antibodies. D and E, Western blots as described in C were probed with anti-phospho-p70 S6 kinase, -p70 S6 kinase, -phospho-4E-BP1, and -4E-BP1 antibodies.