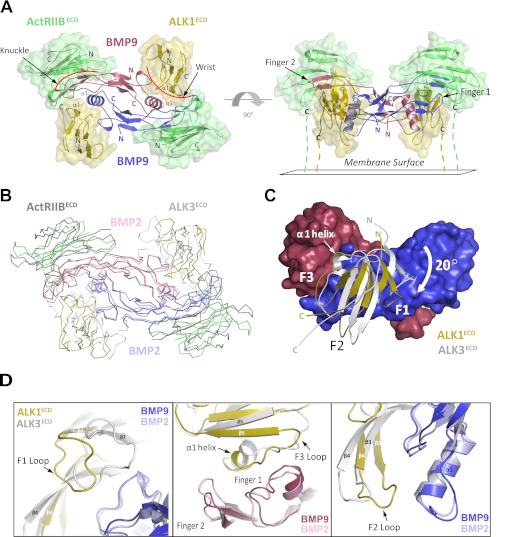
FIGURE 3.
Structure of the ALK1ECD-BMP9-ActRIIBECD ternary complex. A, the ternary complex comprised of one BMP9 homodimer (red/blue), two ALK1ECD receptors (gold), and two ActRIIBECD receptors (green). The complex is shown as viewed from above (left) and from the side with the membrane below (right). B, superposition with the BMP2-ALK3ECD-ActRIIBECD ternary complex (shown in ribbon) via the ligands reveals differences in receptor positioning (BMP2 homodimer colored pink/light blue; ALK3ECD and ActRIIBECD receptors colored light gray and dark gray, respectively; ALK1ECD-BMP9-ActRIIBECD complex colored as in panel A). C, superposition of ALK1ECD and ALK3ECD at the BMP9 interface (shown in surface representation) reveals a 20° rotation of the ALK1 protomer pivoted around helix α1. D, close-up view of helix α1, F1, F2, and F3 loops from ALKECD and ALK3ECD (shown as schematic). Repositioning of these elements in ALK1 results in a novel set of receptor-ligand interactions.