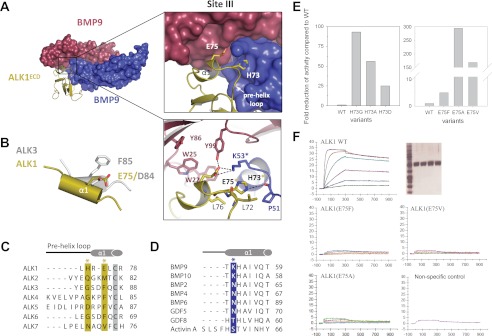
FIGURE 5.
Site III interactions at the ALK1/BMP9 interface. A, close-up of the interaction surface, with color scheme retained from Fig. 3. ALK1 specificity residues His73 and Glu75 and Lys53 from BMP9 are highlighted, with polar contacts drawn as dashed lines. B, overlay of the α1 helix from ALK1 and ALK3 showing the positioning of Glu75 (ALK1) with respect to Asp84 (ALK3) and Phe85 (ALK3). C, sequence alignment of type I receptors (ALK1–7), with ALK1 residues His73 and Glu75 highlighted in gold. D, sequence alignment of BMP9 and BMP10 with select TGF-β ligands, showing the invariant lysine residue Lys53 (blue). Asterisks coincide with panel A. E, cell based, and F, SPR analysis of ALK1 mutants showing effects of His73 and Glu75 substitutions on BMP9 binding and signaling. Proteins were expressed in COS-1 cells, purified by affinity chromatography and showed similar purity (SDS-PAGE given in the upper right panels shows: MW markers (first lane), ALK1-WT (second lane), ALK1(E75F) (third lane), ALK1(E75V) (fourth lane), and ALK1(E75A (fifth lane). SPR analysis was performed with Biacore T100 at 37 °C. Proteins were captured on ani-hFC IgG chip at similar levels. Positive control (WT ALK1) and negative control (unrelated protein that does no bind to BMP9) are given for comparison.