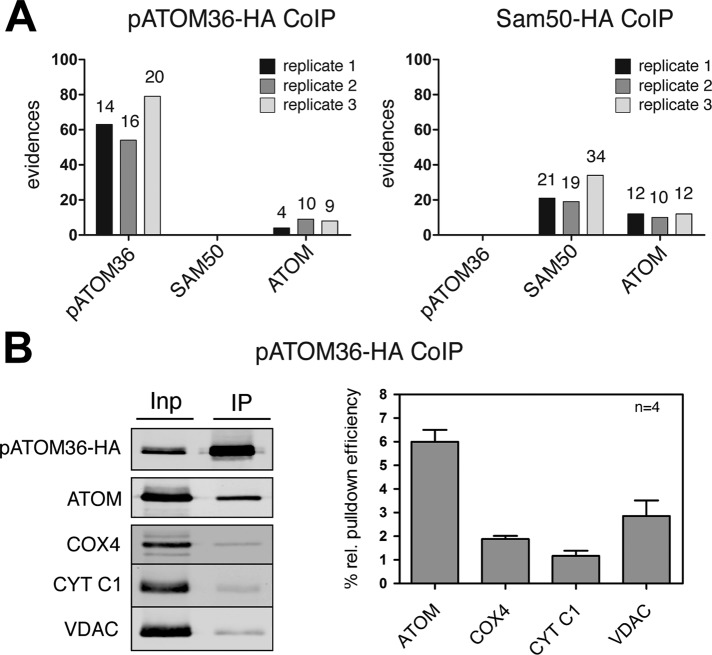
FIGURE 3:
pATOM36 is a peripheral component of the ATOM complex. (A) A 0.15% digitonin lysate of isolated, HA-tagged pATOM36 (left) and HA-tagged SAM50 containing mitochondria (right) were immunoprecipitated using anti-HA antibodies. The corresponding eluates were analyzed for the presence of pATOM36, SAM50, and ATOM using mass spectrometry. The graphs depict the number of evidences that were detected in the eluate of each experiment. The number of unique peptides identified for each protein is depicted on the top of each column. The experiment was performed in triplicate, and immunoprecipitations using wild-type mitochondria lacking any tagged proteins served as controls. pATOM36, SAM50, nor ATOM was recovered in the eluates of wild-type mitochondria. The only exception was ATOM that was detected by a single peptide/evidence in the first replicates of wild-type immunoprecipitates (data not shown). (B) A 0.15% digitonin lysate of isolated, HA-tagged pATOM36–containing mitochondria was immunoprecipitated using anti-HA antibodies and analyzed by immunoblots. Five percent of the total extract (Inp) and 95% of the bound fraction (IP) were analyzed by immunoblot using a monoclonal anti-HA antibody. The same samples were also analyzed with polyclonal ATOM, VDAC, COX4, and CYT C1 antisera. The experiment shown on the left was performed in quadruplicate and quantitated using the Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE). The means of the relative amounts of ATOM, VDAC, COX4, and CYT C1 that were recovered in the bound fraction were calculated. The pATOM36 that was recovered in the pellet was set to 100%. Standard errors are indicated.