FIGURE 1:
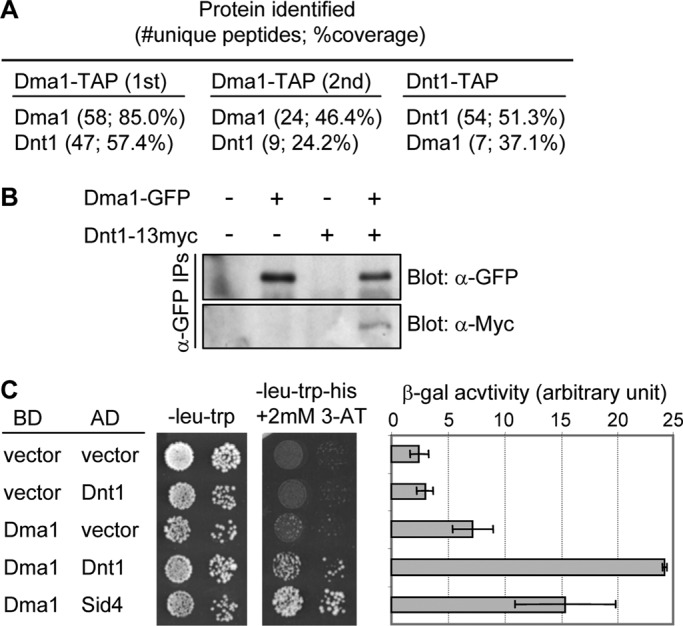
Identification of Dnt1 as a Dma1-binding protein. (A) Results of tandem mass spectrometry analysis of protein mixtures from two independent Dma1-TAP purifications (1st and 2nd) and from one Dnt1-TAP purification. (B) Confirmation of the physical association between Dma1 and Dnt1 in vivo. Lysates were prepared from unsynchronized yeast cells expressing no tags, either Dma1-GFP or Dnt1-13myc, or both Dma1-GFP and Dnt1-13myc. Dma1-GFP was immunoprecipitated, and samples were analyzed by immunoblotting using anti-GFP and anti-Myc antibodies as indicated. (C) Dma1 interacts with Dnt1 by yeast two-hybrid assay. Dma1 was fused with the DNA-binding domain of GAL4 (BD) and Dnt1 with the transcriptional activation domain of GAL4 (AD). S. cerevisiae host strain PJ69-4A was cotransformed with plasmids as indicated, and growth on synthetic defined medium/−Leu, −Trp and synthetic defined medium/−Leu, −Trp, −His, +2 mM 3-aminotriazole is shown (left). As controls, coexpressions of Dma1 and empty AD vector and of empty BD vector with Dnt1 (negative control) or an AD fusion with S. pombe Sid4 (positive control) are shown. The two-hybrid interaction between Dma1 and Sid4 has been shown previously (Guertin et al., 2002b). Mean β-galactosidase activity units from liquid β-galactosidase assay are also shown (right). Error bars, SD from three independent experiments.
