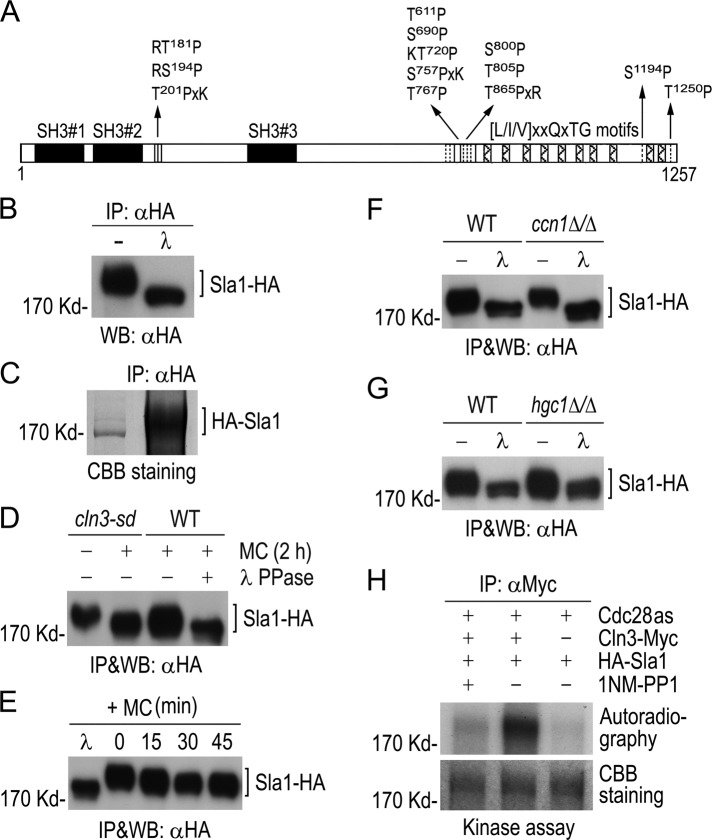
FIGURE 2:
Phosphorylation of Sla1 by Cdc28−Cln3. (A) Schematic diagram of Sla1 showing CDK phosphorylation sites (vertical lines). Solid boxes indicate SH3 domains, and hatched boxes show putative Prk1 kinase recognition motifs. (B) Detection of phosphorylated Sla1 in vivo. Sla1-HA was immunoprecipitated from GZY584 (SLA1-HA) yeast cell lysate and subjected to λPP or mock treatment, followed by αHA WB analysis. (C) Purification of Sla1 for phospho-site mapping. HA-Sla1 was immunoprecipitated from YPG culture of GZY631 (PGAL1-HA-SLA1) yeast cells and electrophoresed on SDS–PAGE. After staining with CBB, the Sla1 band was excised for phospho-site mapping by MS. (D) Cln3-dependent phosphorylation of Sla1 in vivo. GMM culture of cln3-sd yeast cells expressing Sla1-HA (GZY622) was incubated with 0.5 mM Met and Cys (MC) at 30°C for 2 h to switch off CLN3 expression. Sla1-HA was immunoprecipitated from cell lysates for WB analysis. A WT strain (BWP17) expressing Sla1-HA (GZY584) was included as a control, and half of the immunoprecipitated Sla1-HA was treated with λPP. (E) Sla1 phosphorylation in Cln3-depleted cells. GZY622 cells were grown in GMM in the presence of 0.5 mM MC at 30°C, and samples were taken at 15-min intervals for IP and WB analysis of Sla1-HA using αHA. Part of Sla1-HA from the 0-min time point was treated with λPP for control. (F) Comparison of Sla1 phosphorylation between ccn1Δ/Δ and WT cells. Sla1-HA was immunoprecipitated from lysates of WT (GZY584) and ccn1Δ/Δ (GZY744) yeast cells and subjected to λPP or mock treatment for WB analysis. (G) Comparison of Sla1 phosphorylation between hgc1Δ/Δ and WT cells. WT (GZY584) and hgc1Δ/Δ (GZY624) yeast cells were induced with 20% FBS at 37°C for 2 h for hyphal growth. Sla1-HA was then immunoprecipitated and subjected to λPP or mock treatment for WB analysis. (H) In vitro phosphorylation of Sla1 by Cdc28−Cln3. Cln3-Myc was immunoprecipitated from GZY641 (cdc28as CLN3-Myc) yeast cells and incubated with immunopurified HA-Sla1 to perform in vitro kinase assay in the presence or absence of 25 μM 1NM-PP1. Immunoprecipitates from IS89 (cdc28as) cell lysates were used as a negative control. After electrophoresis, the gel was stained with CBB to visualize HA-Sla1 and dried onto a filter paper for autoradiography.