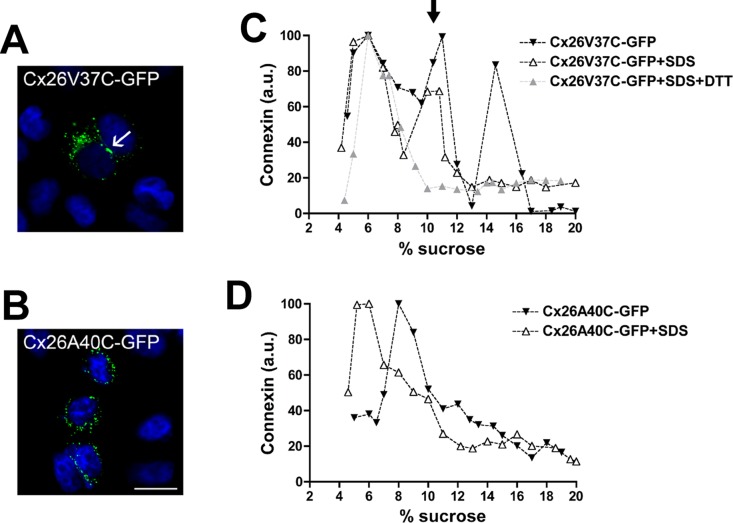
FIGURE 5:
Replacement of Val-37 (but not Ala-40) with cysteine allows disulfide bond formation and stabilization of intermediate oligomers. (A and B) Photomicrographs show the distribution of GFP fluorescence in HeLa cells transfected with Cx26V37C-GFP (A) and Cx26A40C-GFP (B). Nuclei were stained with 4′,6-diamidino-2-phenylindole. The arrow in (A) points to a gap junction plaque. Scale bar: 30 μm. (C and D) Graphs represent the levels of Cx26V37C-GFP (C) and Cx26A40C-GFP (D) in sucrose gradient fractions detected by immunoblotting using anti-Cx26 antibodies. Triton X-100–soluble extracts from HeLaCx26V37C-GFP or HeLaCx26A40C-GFP cells left untreated (closed symbols) or treated only with SDS (open triangles) or with DTT and SDS (gray triangles) were subjected to sedimentation velocity through 5–20% sucrose gradients. Levels of connexin are presented in arbitrary units (a.u.) after densitometric analysis of the respective immunoblots. The arrow in (C) indicates the intermediate oligomer peak (likely dimers).