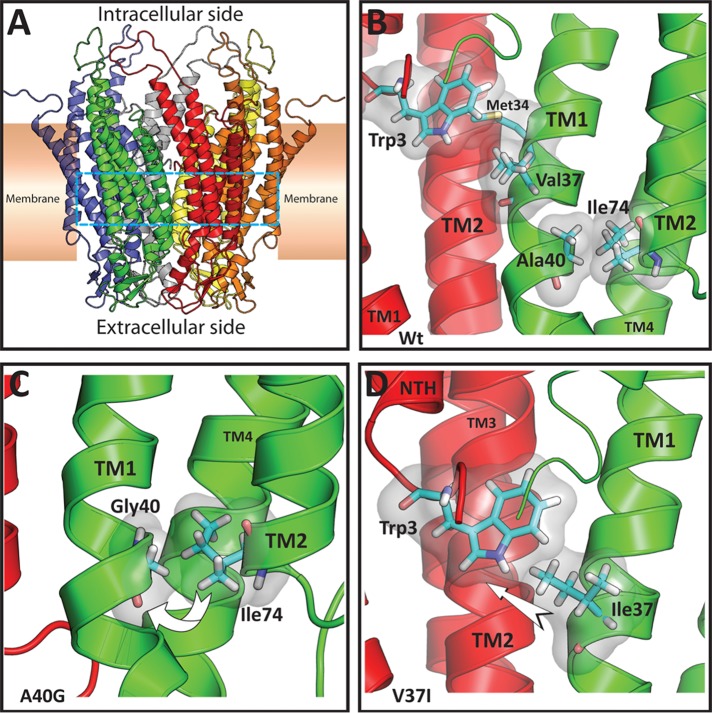
FIGURE 8:
Structural models of rat Cx26 and the nonsyndromic deafness-associated mutants V37I and A40G. (A) Ribbon representation of the overall structure of the Cx26 hemichannel colored by monomer. The cyan dashed lines demarcate the location of the residues shown in detail in (B–D). (B) View from inside the pore of the Cx26 hemichannel illustrating the main interactions of residues Val-37 and Ala-40 in two protomers shown in ribbon representation (red and green). For clarity, the amino terminus (NTH) of the protomer shown in green is not shown. Relevant residues are shown in Licorice representation colored by element (C = cyan, N = blue, O = red, H = white, S = yellow) and surrounded by a transparent surface. Val-37 is on the same face (but approximately one turn away) of the TM1 α-helix as Met-34 which interacts with Trp-3 from an adjacent protomer. Ala-40 is involved in a TM1–TM2 intraprotomer interaction with Ile-74. (C) Detail of the Cx26A40G model. It is possible that replacement of Ala-40 by glycine might decrease the interaction with Ile-74, perhaps increasing the mobility of TM1 (curved arrow). (D) Detail of the Cx26V37I model. It is possible that the increased size and volume of the isoleucine residue at position 37 (TM1 from the protomer shown in green) might enhance the interaction of this residue with Trp-3 of the adjacent monomer (NTH from the adjacent protomer shown in red), perhaps leading to movement of the amino terminal helix (arrow).