Collagen/β1 integrin/extracellular signal-regulated kinase signaling up-regulates the expression and function of ABCC1 transporter. This suggests that its activation could represent an important pathway in cancer chemoresistance.
Abstract
The mechanisms by which β1 integrins regulate chemoresistance of cancer cells are still poorly understood. In this study, we report that collagen/β1 integrin signaling inhibits doxorubicin-induced apoptosis of Jurkat and HSB2 leukemic T-cells by up-regulating the expression and function of the ATP-binding cassette C 1 (ABCC1) transporter, also known as multidrug resistance–associated protein 1. We find that collagen but not fibronectin reduces intracellular doxorubicin content and up-regulates the expression levels of ABCC1. Inhibition and knockdown studies show that up-regulation of ABCC1 is necessary for collagen-mediated reduction of intracellular doxorubicin content and collagen-mediated inhibition of doxorubicin-induced apoptosis. We also demonstrate that activation of the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase signaling pathway is involved in collagen-induced reduction of intracellular doxorubicin accumulation, collagen-induced up-regulation of ABCC1 expression levels, and collagen-mediated cell survival. Finally, collagen-mediated up-regulation of ABCC1 expression and function also requires actin polymerization. Taken together, our results indicate for the first time that collagen/β1 integrin/ERK signaling up-regulates the expression and function of ABCC1 and suggest that its activation could represent an important pathway in cancer chemoresistance. Thus simultaneous targeting of collagen/β1 integrin and ABCC1 may be more efficient in preventing drug resistance than targeting each pathway alone.
INTRODUCTION
Integrins are α/β membrane receptors that mediate cell–cell interactions and cell adhesion to the surrounding extracellular matrix (ECM). Among these receptors, the β1 integrin subfamily is widely expressed and constitutes a major class of integrins that mediate cell interactions with ECM components, including fibronectin, laminins, and collagens (Humphries et al., 2006; Harburger and Calderwood, 2009). In addition to their role in cell adhesion, integrins also activate several intracellular signaling pathways, such as focal adhesion kinase, integrin-linked kinase, Src kinases, and the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) and phosphoinositide (PI) 3-kinase/Akt pathways, which modulate cell growth and differentiation, migration, and survival (Lee and Juliano, 2004; Harburger and Calderwood, 2009; Streuli, 2009). In cancer, the prosurvival signals mediated by integrin–ECM interactions led in several cases to the resistance of tumor cells to chemotherapeutic drug-induced apoptosis (Hehlgans et al., 2007; Meads et al., 2009).
Several studies have shown that integrins can protect both solid and hematological malignancies from chemotherapy-induced apoptosis by up-regulating antiapoptotic proteins and/or down-modulating the proapoptotic proteins of the Bcl-2 family, as well as inhibiting activation of the caspase cascade (Hehlgans et al., 2007; Meads et al., 2008, 2009). In addition to the direct modulation of apoptotic events, resistance to chemotherapy can also result from several cell adaptations (Luqmani, 2005; Fodale et al., 2011). Indeed, the treatment efficiency could be altered in resistant malignancies by overexpressing proteins that metabolize (Roos and Bolt, 2005) or chelate (Ruzza et al., 2009) the drug more efficiently. Resistant cancer cells could also escape from chemotherapy by decreasing the drug concentrations in the cytoplasm by expressing several transporters that belong to the ATP-binding cassette (ABC) superfamily (Deeley et al., 2006; Chen and Tiwari, 2011). These proteins, through the hydrolysis of ATP, have the ability to actively promote cell detoxification by creating unidirectional flow of a wide group of endogenous (steroids, metabolites, ions) or exogenous substrates (drugs) from the cytosol to the extracellular milieu. Drug transporters of the ABC family play a central role in the development of multidrug resistance (MDR), a phenomenon in which cancer cells acquire cross-resistance to an array of different chemotherapeutic compounds (Luqmani, 2005; Deeley et al., 2006; Fodale et al., 2011). MDR-1, also called ABCB1 or P-glycoprotein, is the first member of the ABC transporters to have been described (Sharom, 2011). ABCB1 and another ABC transporter, ABCC1, also called multidrug resistance–associated protein-1 (MRP-1), are the most studied ABC transporters and are expressed throughout normal and malignant tissues (Chen and Tiwari, 2011; Sharom, 2011).
The signaling pathways regulating the activity and the high expression levels of ABC transporters in malignant cells are not fully understood. In this study, we asked whether ECM–integrin signaling could regulate ABC transporters. We tested this possibility in leukemic T-cell lines in which we previously showed that collagen, via its integrin receptors, was able to reduce doxorubicin-induced apoptosis (Gendron et al., 2005). We show that collagen increased ABCC1 expression in an ERK-dependent manner in leukemic T-cells, which consequently decreased the amount of intracellular doxorubicin and doxorubicin-induced apoptosis. Our results provide the first evidence of the regulation of ABCC1 by integrin signaling in cancer chemoresistance and suggest that simultaneous targeting of collagen/β1 integrin and ABCC1 might be more efficient in preventing drug resistance than targeting each pathway alone.
RESULTS
Collagen, but not fibronectin, reduces intracellular doxorubicin content
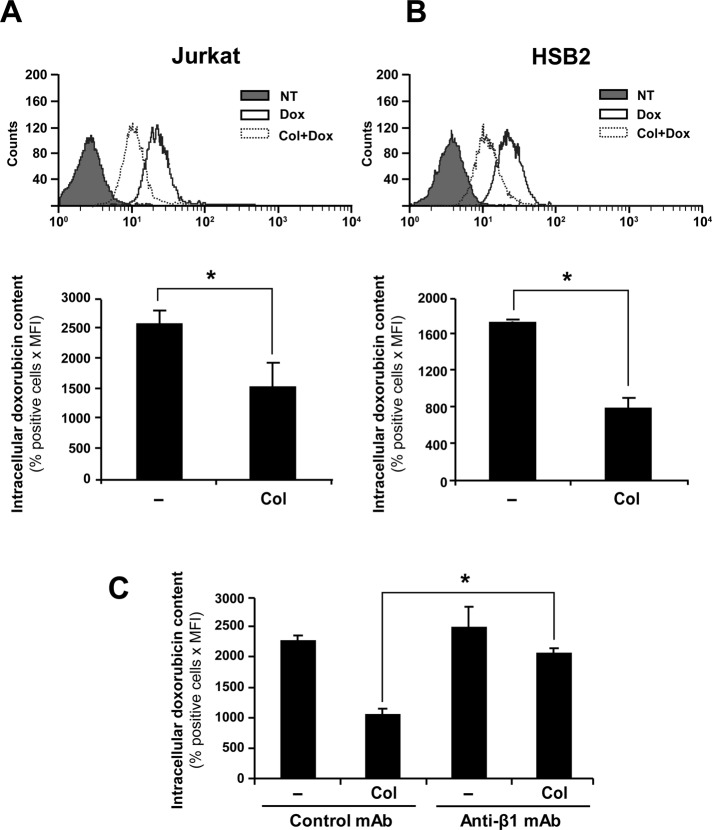
To determine whether ECM regulates the amount of intracellular doxorubicin, we studied the effect of collagen and fibronectin on doxorubicin accumulation in Jurkat and HSB2 T-cell acute lymphoblastic leukemia (T-ALL) cell lines. We show that preactivating Jurkat (Figure 1A) and HSB2 cells (Figure 1B) with collagen reduces the intracellular accumulation of doxorubicin as determined by flow cytometry. Quantitative analysis indicates that the intracellular doxorubicin amount is reduced in collagen-treated cells by 50–65% in comparison to nontreated cells (Figure 1, A and B, bottom). Treatment of the cells with the collagen diluent had no effect on doxorubicin intracellular accumulation (unpublished data). The effect of collagen on amount of intracellular doxorubicin is also observed in cells treated with doxorubicin for 4 and 24 h (Supplemental Figure S1).
FIGURE 1:
Collagen/β1 integrin reduces intracellular amounts of doxorubicin. Jurkat (A) and HSB2 (B) cells were preactivated or not with collagen (Col) for 4 h and then treated with 250 ng/ml doxorubicin (Dox) for an additional 2 h at 37°C in the dark. The cells were then washed with PBS, and the intracellular doxorubicin content was analyzed by flow cytometry using the FL-2 settings. Top, flow cytometric profile of intracellular doxorubicin content (NT, nontreated; cells that had not been treated with doxorubicin). Intracellular doxorubicin content (bottom) was quantified according to the following formula: positive cells (%) times mean fluorescence intensity (MFI). (C) Jurkat cells were pretreated with 10 μg/ml anti-β1 (anti-β1 mAb) or IgG isotypic control antibody (control mAb) for 1 h before being activated with collagen. The cells were then treated with doxorubicin, and intracellular doxorubicin amount was determined as described. The results presented in A and B (bottom) and in C represent mean values ± SD from three independent experiments. *p < 0.05 where indicated.
The β1 integrins are the major collagen receptors expressed by leukemic T-cell lines (Chan et al., 1991; Aoudjit and Vuori, 2000; Ivanoff et al., 2005; Van de Walle et al., 2005). Thus we examined whether the collagen effect is mediated via β1 integrin receptors. As shown in Figure 1C, the effect of collagen on the reduction of intracellular doxorubicin content is reversed by the anti–β1 integrin blocking antibody but not by the isotypic control antibody. In addition, the anti–β1 integrin antibody did not affect the accumulation of intracellular doxorubicin in the absence of collagen. Together these results indicate that collagen reduces the intracellular doxorubicin content via β1 integrins.
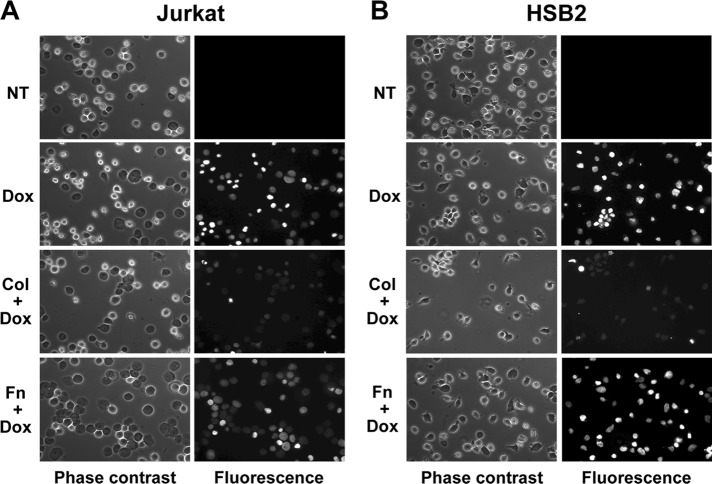
In contrast to collagen, fibronectin did not regulate the intracellular doxorubicin content in Jurkat (Figure 2A) and HSB2 T-cell lines (Figure 2B). In addition, the differential effect of collagen and fibronectin was also confirmed by fluorescence microscopy (Figure 3). Indeed, collagen but not fibronectin reduces the fluorescence intensity of cells exposed to doxorubicin. Taken together, these results indicate that collagen could be the main matrix protein that reduces the amount of intracellular doxorubicin in T-ALL cell lines.
FIGURE 2:
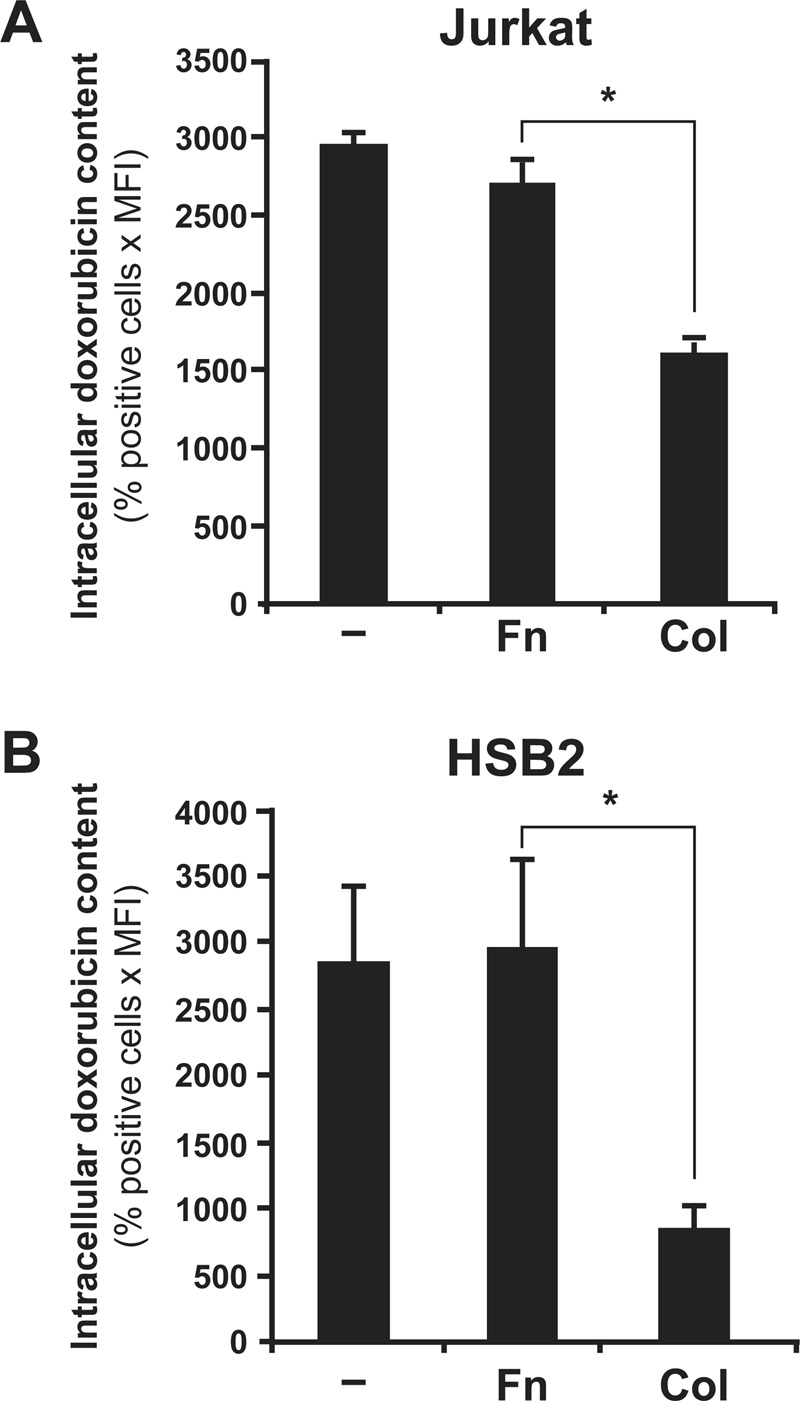
Fibronectin had no effect on intracellular doxorubicin content. Jurkat (A) and HSB2 (B) cells were preactivated or not with collagen (Col) or fibronectin (Fn) before the addition of doxorubicin. The cells were then washed with PBS, and the intracellular doxorubicin content was quantified by flow cytometry. The results represent mean values ± SD from three independent experiments. *p < 0.05 where indicated.
FIGURE 3:
Collagen but not fibronectin also reduces intracellular doxorubicin content as measured by fluorescence microscopy. Jurkat (A) and HSB2 (B) cells were preactivated with collagen (Col), fibronectin (Fn), or left unstimulated (NT) for 4 h and then were treated or not with doxorubicin (Dox) for 2 h. The cells were washed, fixed, and mounted between slides and cover slips and were observed by phase contrast and by using a red excitation fluorescence filter. The results are representative of three independent experiments.
Collagen increases the expression of ABCC1
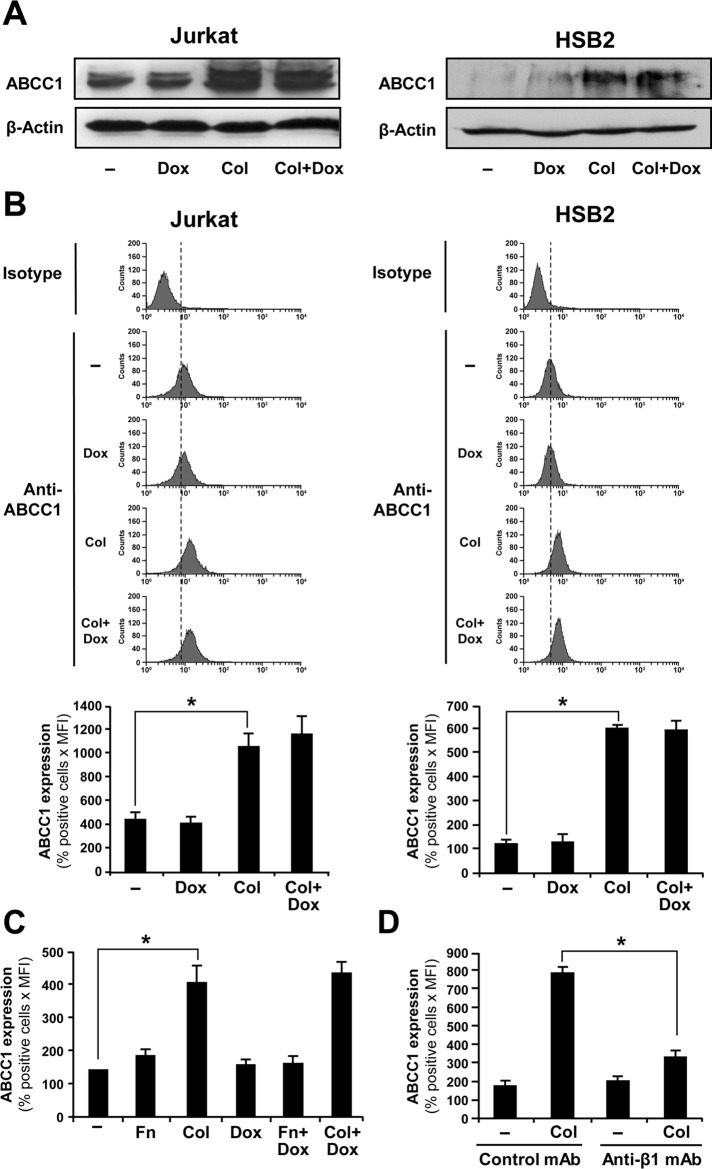
Doxorubicin is a ligand for several ABC transporter family members, including ABCC1, ABCC2, ABCC6, ABCB1, and ABCG2 (Belinsky et al., 2002; Luqmani, 2005; Deeley et al., 2006; An and Ongkeko, 2009). In addition, the ability of ABC transporters to clear their ligands from the intracellular milieu could be regulated by their expression levels (Bonhoure et al., 2006; Angelini et al., 2007). Accordingly, we studied the expression and the regulation of these drug transporters by collagen in T-ALL cell lines. Previous studies reported that ABCC1 is the major ABC family member expressed in leukemic T-cell lines, including Jurkat cells (Martel et al., 1997; van der Heijden et al., 2004; Franco and Cidlowski, 2006; Hammond et al., 2007). Similarly, we found that Jurkat and HSB2 cells express basal mRNA levels of ABCC1 but not of ABCC2, ABCC6, ABCB1, and ABCG2 transporters (Figure 4). Treatment of the cells with collagen up-regulates the ABCC1 mRNA levels but had no effect on the expression of other ABC transporters (Figure 4). As a positive control, and as previously shown (Gutmann et al., 1999, 2005; Prime-Chapman et al., 2004), we found that the CaCo2 colorectal cancer cells express significant levels of ABCC2, ABCC6, ABCB1, and ABCG2 and weak/low levels of ABCC1 mRNA (Figure 4). Doxorubicin treatment, which was previously reported to regulate ABC transporters levels (Steinbach et al., 2006), had no effect on the mRNA levels of the ABC transporters (Figure 4). We then examined the expression of ABCC1 at the protein level. Immunoblot analysis shows that collagen up-regulates ABCC1 levels in both Jurkat and HSB2 cells (Figure 5A). In addition, flow cytometry analysis revealed that collagen treatment increased ABCC1 expression in Jurkat and HSB2 cells by threefold and fivefold, respectively (Figure 5B). Similar to the mRNA analysis, doxorubicin did not modulate the ABCC1 protein levels (Figure 5, A and B).
FIGURE 4:
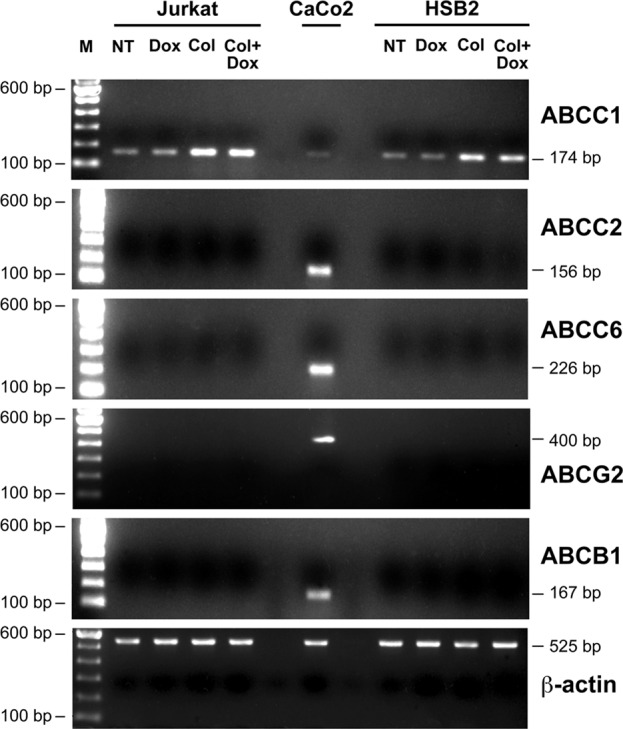
ABCC1 is the major ABC transporter expressed in leukemic T-cell lines. Jurkat and HSB2 cells were preactivated with collagen (Col) or left unstimulated (NT) for 4 h and were then treated with doxorubicin (Dox) for 2 h. mRNA levels for the different ABC transporters and for β-actin were determined by reverse transcriptase-PCR using specific primers (Table 1). The CaCo2 cell line was used as a positive control for ABCC2, ABCC6, ABCB1, and ABCG2 mRNA detection. M, the 100–base pair DNA ladder marker. The results are representative of three independent experiments.
FIGURE 5:
Collagen but not fibronectin up-regulates ABCC1 protein levels through β1 integrin. (A) Immunoblot analysis of ABCC1 expression. Jurkat (left) and HSB2 (right) cells were preactivated with collagen (Col) for 4 h and then treated with 250 ng/ml doxorubicin (Dox) for 2 h. The cells were lysed and the cell lysates analyzed by immunoblot using the anti-ABCC1 mAb. The blots were stripped and reprobed with control anti–β-actin antibody to ensure equal loading. The results are representative of two independent experiments. (B) Flow cytometry analysis of ABCC1 expression in Jurkat and HSB2 cells. After collagen activation and doxorubicin treatment, the cells were washed with PBS, and ABCC1 expression was assessed by flow cytometry by staining the cells with FITC-coupled anti-ABCC1 antibody or appropriate FITC-conjugated isotypic antibody. Top, representative flow cytometric profiles of ABCC-1 expression. Bottom, quantification of ABCC-1 expression. (C) Fibronectin had no effect on ABCC1 levels. Jurkat cells were preactivated with collagen (Col) or fibronectin (Fn) and then treated or not with doxorubicin (Dox). The cells were then washed with PBS, and ABCC1 expression was assessed by flow cytometry. (D) Collagen-mediated up-regulation of ABCC1 levels is dependent on β1 integrin. Jurkat cells were pretreated with the control or anti–β1 integrin blocking antibodies for 1 h and then activated or not with collagen (Col), and ABCC1 expression was assessed by flow cytometry. The data in B (bottom), C, and D represent mean values ± SD of positive cells (%) times the mean fluorescence intensity from three independent experiments. *p < 0.05 where indicated.
The increased of ABCC1 protein levels were maintained for at least 12 h after the addition of collagen (Supplemental Figure S2). We also found that fibronectin, which had no effect on intracellular doxorubicin content, failed to up-regulate ABCC1 expression (Figure 5C). The collagen effect on ABCC1 expression is mediated through β1 integrins, since it was reversed by the blocking anti–β1 integrin monoclonal antibody, which alone had no effect on ABCC1 expression (Figure 5D). Taken together, these results indicate that ABCC1 is the major transporter expressed in leukemic T-cell lines and that collagen, but not fibronectin, could enhance ABCC1 activity by up-regulating its expression levels in T-ALL cell lines via β1 integrin signaling.
The reduction of intracellular doxorubicin content and doxorubicin-induced apoptosis by collagen involves the ABCC1 drug transporter
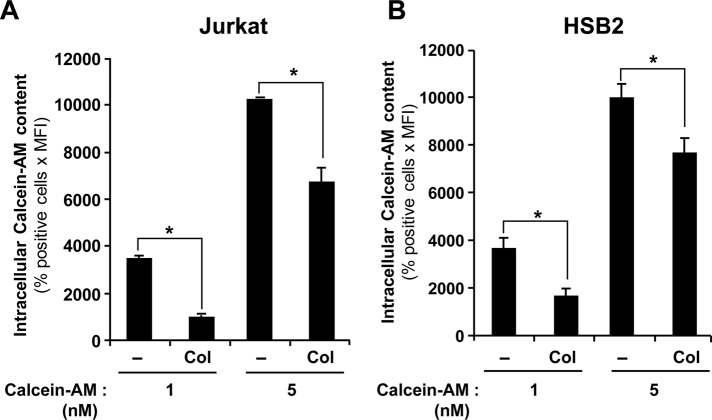
Because collagen treatment strongly up-regulated the expression of ABCC1 in T-ALL cells, we studied the potential implication of this transporter in the collagen-induced inhibition of intracellular doxorubicin content. As a first step, we tested whether collagen would reduce the intracellular accumulation of calcein-AM, a molecule that could be transported by ABCC1 (Lebedeva et al., 2011). The results show that collagen significantly reduces the amount of intracellular calcein-AM in Jurkat (Figure 6A) and HSB2 cells (Figure 6B), thus supporting the implication of ABCC1.
FIGURE 6:
Collagen down-regulates the intracellular accumulation of calcein-AM. Jurkat (A) and HSB2 (B) cells were preactivated or not with collagen (Col) for 4 h and then treated with increasing concentrations of calcein-AM for 30 min at 37°C in the dark. The cells were washed with PBS, and the intracellular calcein-AM content was analyzed by flow cytometry using the FL-1 settings. The results represent mean values ± SD of positive cells (%) times the mean fluorescence intensity from three independent experiments. *p < 0.05 where indicated.
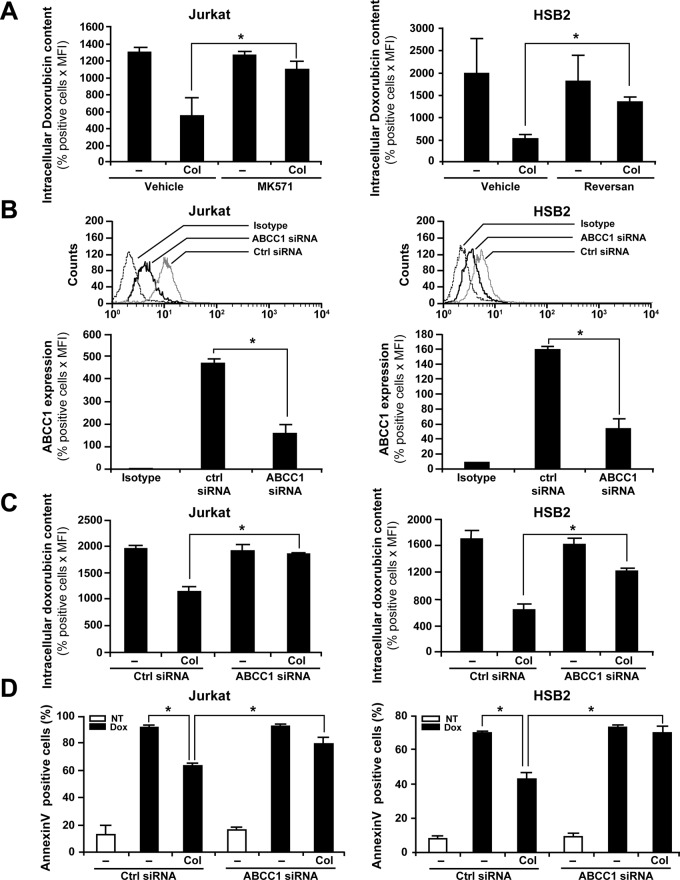
To test the potential role of ABCC1 in collagen-induced doxorubicin efflux, we used pharmacological and genetic inhibitory strategies. Treatment of Jurkat cells with MK571, a frequently used ABCC1 inhibitor (Abdul-Ghani et al., 2006; Hammond et al., 2007), but not with the vehicle, significantly reduced the ability of collagen to decrease the content of intracellular doxorubicin (Figure 7A, left). We also used Reversan, a more recently described ABCC1 inhibitor (Burkhart et al., 2009). This compound also strongly abrogates the collagen-induced doxorubicin efflux in HSB2 cells (Figure 7A, right). The effect of the two inhibitors was not due to cytotoxicity, as we found no difference in cell viability between control and treated cells (95–96%).
FIGURE 7:
ABCC1 inhibition abrogates collagen-induced drug efflux and protection against doxorubicin-mediated apoptosis. (A) ABCC1 inhibitors reduce collagen-mediated doxorubicin efflux. Jurkat (left) and HSB2 (right) cells were pretreated for 1 h with 20 μM of MK571 or Reversan, respectively, or with the vehicle. The cells were then activated or not with collagen (Col) and treated with doxorubicin. The cells were washed with PBS, and intracellular doxorubicin content was analyzed by flow cytometry using the FL-2 settings. (B) ABCC1 siRNA reduces ABCC1 levels. Jurkat and HSB2 cells were transfected with ABCC1 or control (Ctrl) siRNAs as described in Materials and Methods. Efficiency of ABCC1 silencing in Jurkat and HSB2 cells was monitored by flow cytometry analysis of ABCC1 expression levels using FITC-coupled anti-ABCC1 mAb as described in Materials and Methods. Control isotypic staining of the cells is shown. Bottom, quantification of ABCC1 expression in control and in ABCC1 siRNA-transfected cells. (C) ABCC1 siRNA reduces collagen-mediated doxorubicin efflux. Transfected Jurkat and HSB2 cells were activated or not with collagen (Col) before their treatment with doxorubicin. The cells were washed with PBS, and intracellular doxorubicin content was assessed by flow cytometry. (D) ABCC1 siRNA reverses the protective effect of collagen on doxorubicin-induced apoptosis. Transfected Jurkat and HSB2 cells were left untreated (NT) or activated with collagen (Col) for 4 h and then treated with doxorubicin (Dox). After 16 h of drug treatment, apoptosis was determined by annexin V staining and flow cytometry. The results in the different panels represent mean values ± SD from three independent experiments. *p < 0.05 where indicated.
The implication of ABCC1 was also studied by a small interfering RNA (siRNA) knockdown approach. A specific ABCC1 siRNA markedly reduced the transporter levels in Jurkat and HSB2 cells as monitored by flow cytometry (Figure 7B). Quantification analysis indicates that the ABCC1 levels were reduced by ∼60% (Figure 7B, bottom). The reduction in ABCC1 protein levels is confirmed by immunoblot analysis (Supplemental Figure S3A). The ABCC1 siRNA also reduces ABCC1 levels in collagen-treated cells (Supplemental Figure S3B). Silencing ABCC1 significantly diminished the ability of collagen to reduce the intracellular accumulation of doxorubicin in Jurkat and HSB2 cells in comparison with control siRNA-transfected cells (Figure 7C). Together these results demonstrate that the effect of collagen on intracellular doxorubicin content involves ABCC1.
We previously reported that collagen protects T-ALL cell lines from doxorubicin-induced apoptosis (Gendron et al., 2005). Thus we tested whether the reduction of intracellular doxorubicin content by ABCC1 could contribute to collagen-induced T-ALL cell survival. To this end, we determined whether silencing ABCC1 affects the prosurvival effect of collagen. ABCC1 silencing had no effect on spontaneous apoptosis but strongly abrogated the ability of collagen to reduce apoptosis induced by a 16-h treatment with doxorubicin in Jurkat and HSB2 cells (Figure 7D). ABCC1 siRNA had a similar effect when apoptosis was measured after 48 h of doxorubicin treatment (Supplemental Figure S4), further supporting the role of ABCC1 in collagen-induced doxorubicin resistance. Taken together, these results indicate that collagen inhibits doxorubicin-induced apoptosis by reducing its intracellular accumulation via a mechanism involving ABCC1 function.
ERK signaling is required for collagen-induced effects on ABCC1 activity, expression, and cell survival
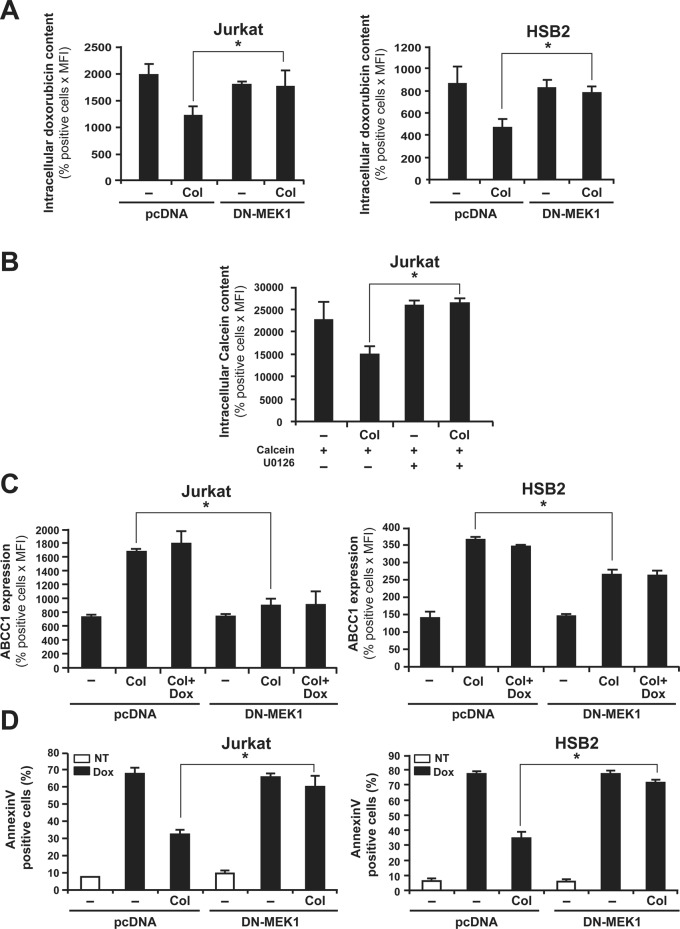
Integrin signaling leads to the activation of two major cell survival pathways, including the ERK/MAPK and PI 3-kinase/Akt pathways. We previously showed that collagen increases the activation of the ERK/MAPK pathway but not that of the PI 3-kinase/Akt pathway in T-ALL cell lines (Gendron et al., 2003; Chetoui et al., 2006). Therefore we examined the potential effect of ERK inhibition on collagen-induced ABCC1 function. As shown in Figure 8, expression of a flagged dominant-negative form of MEK-1 (DN-MEK-1) in Jurkat and HSB2 cells (Figure 8A) abrogated the collagen-induced reduction of intracellular doxorubicin amounts in comparison to cells transfected with the control vector (pcDNA). As a control, DN-MEK-1– but not pcDNA-transfected cells express the FLAG-DN-MEK-1 (Supplemental Figure S5). In addition, we found that the specific MEK/ERK inhibitor U0126 reversed collagen-induced calcein-AM efflux (Figure 8B). These results indicate that ERK/MAPK is required for collagen-induced inhibition of intracellular drug accumulation. We then explored whether the ERK signaling pathway regulates ABCC1 expression. The results show that DN-MEK-1–expressing Jurkat and HSB2 cells exhibit reduced ABCC1 levels in response to collagen when compared with control cells (Figure 8C). Finally, DN-MEK-1 also abrogated the protective effect of collagen on doxorubicin-induced apoptosis (Figure 8D). Taken together, our results indicate that collagen-induced ERK signaling in T-ALL cell lines renders them less sensitive to doxorubicin-induced apoptosis by increasing the expression levels of ABCC1, which reduces the amount of intracellular doxorubicin.
FIGURE 8:
ERK inhibition abrogates collagen-induced drug efflux, ABCC1 expression, and resistance against doxorubicin-mediated apoptosis. (A) DN-MEK-1 inhibits the ability of collagen to reduce intracellular doxorubicin content. Cells were transfected with DN-MEK1 or pcDNA (empty plasmid). After transfection, the cells were activated or not with collagen (Col) and then treated with doxorubicin. The intracellular doxorubicin content was measured by flow cytometry. (B) The MEK-1 inhibitor abrogates collagen-mediated calcein efflux. Jurkat cells were preincubated or not for 1 h with 10 μM of the MEK/ERK inhibitor (U0126) before their activation or not with collagen (Col) and treatment with calcein-AM. Intracellular calcein content was then assessed by flow cytometry. (C) DN-MEK-1 abolishes the up-regulation of ABCC1 expression levels by collagen. Expression of ABCC1 in transfected cells was determined by ABCC1 staining and flow cytometry analysis as described in Materials and Methods. (D) DN-MEK-1 abolishes the protective effect of collagen on doxorubicin-induced apoptosis. Transfected cells were activated or not with collagen and then treated or not with doxorubicin (Dox) for 16 h (NT, nontreated). Apoptosis was determined by annexin V staining and flow cytometry analysis. The results represent mean values ± SD from three independent experiments. *p < 0.05 where indicated.
Actin polymerization is required for collagen-induced doxorubicin efflux
Actin polymerization was shown to be involved in collagen-induced ERK activation in Jurkat cells (Bijian et al., 2007) and to be essential for ABCC1 activity (Hummel et al., 2011). Because we found that the effect of collagen on doxorubicin efflux and ABCC1 expression is dependent on ERK activation, we examined whether actin polymerization was also part of collagen signaling in up-regulating doxorubicin efflux and ABCC1 expression levels. We show that treatment of Jurkat cells with collagen substantially enhances the content of polymerized filamentous actin (F-actin) as monitored by Alexa 594–conjugated phalloidin staining and flow cytometry analysis (Figure 9A). This effect was totally abrogated by cytochalasin B (CB), an inhibitor of F-actin formation (Figure 9A). We then tested whether inhibition of actin polymerization affects collagen-induced doxorubicin efflux and ABCC1 expression. We found that CB significantly reduces the inhibitory effect of collagen on intracellular doxorubicin content (Figure 9B) and inhibits the up-regulation of ABCC1 levels by collagen (Figure 9C). In the absence of CB, collagen reduced doxorubicin content by 58%. However, in the presence of CB, collagen reduced doxorubicin content by only 25%. With regard to the up-regulation of ABCC1, CB strongly inhibited the ability of collagen to up-regulate ABCC1 (Figure 9C). The ABCC1 levels in cells treated with collagen plus CB are almost equal to those in non–collagen-treated cells (basal levels). Similar results were also obtained with HSB2 cells (unpublished data). Together these results indicate that collagen-induced up-regulation of ABCC1 expression levels and function requires actin polymerization.
FIGURE 9:
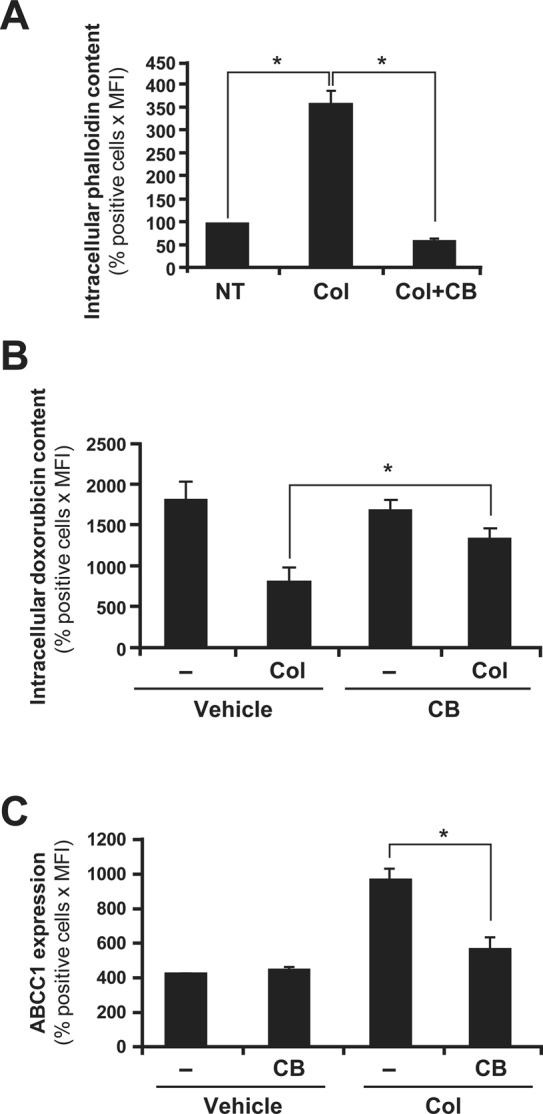
Actin polymerization is required for collagen-induced drug efflux and ABCC1 expression. (A) Cytochalasin B (CB) abrogates collagen-induced F-actin formation. Jurkat cells were left untreated (NT) or activated with collagen (Col) in the presence or absence of CB (10 μM). The F-actin content was assessed by staining with Alexa Fluor 594–conjugated phalloidin (1 μM) and flow cytometry analysis as described in Materials and Methods. (B) Actin cytoskeleton disruption abrogates collagen-induced drug efflux. Jurkat cells were activated or not with collagen (Col) in the presence or absence of CB and then treated with doxorubicin. Intracellular doxorubicin content was then assessed by flow cytometry analysis. (C) Actin cytoskeleton disruption abrogates collagen-induced ABCC1 expression. Jurkat cells were activated or not with collagen (Col) in the presence or absence of CB. ABCC1 expression was then assessed by intracellular staining and flow cytometry as described in Materials and Methods. The results represent mean values ± SD of positive cells (%) times the mean fluorescence from three independent experiments. *p < 0.05 where indicated.
DISCUSSION
One major hurdle in anticancer therapy is the development of drug resistance. Thus understanding the mechanisms contributing to this process is likely to bring new insights into the design of new therapeutic strategies. Interaction of cancer cells with ECM is recognized as a critical factor contributing to their resistance to chemotherapy-induced apoptosis. We previously reported that collagen, via its α2β1 integrin receptor, protected T-ALL cell lines from doxorubicin-induced apoptosis (Gendron et al., 2005). In this study, we demonstrate that collagen inhibits doxorubicin-induced apoptosis by promoting doxorubicin efflux via a mechanism involving activation of the ERK/MAPK and up-regulation of the ABC transporter family member ABCC1. Inhibition and knockdown of ABCC1 with siRNA not only prevented collagen from reducing intracellular doxorubicin content, but it also abolished the protective effect of collagen on doxorubicin-induced apoptosis, indicating that the reduction of intracellular doxorubicin amounts is an event that contributes to the protective effect of collagen.
Doxorubicin is a substrate for ABCC1, which has been associated with doxorubicin resistance in multidrug-resistant cancer cells (Cole et al., 1992; Bonhoure et al., 2006; Angelini et al., 2007). However, our study indicates that sensitive cancer cells, such as the leukemic T-cell lines Jurkat and HSB-2, can diminish their sensitivity to doxorubicin by up-regulating ABCC1. In this context, our study provides the first evidence that ABCC1 expression and activity can be regulated by β1 integrin signaling.
One major growth site for T-ALL and other hematological malignancies is the bone marrow, which is a tissue rich in ECM, such as fibronectin and collagen (Meads et al., 2008). Thus our study suggests that by interacting with collagen, T-ALL cells could up-regulate ABCC1 levels and escape the cytotoxic effect of doxorubicin and subsequently become resistant to doxorubicin. Of interest, high levels of ABCC1 have been shown to be expressed in hematological malignancies, including T-ALL (Martel et al., 1997; Ikeda et al., 1999; Poulain et al., 2000; Consoli et al., 2002; Hammond et al., 2007). Thus our findings suggest an important role for the collagen/β1 integrin signaling pathway in the up-regulation of ABCC1 expression levels in these malignancies and in their resistance to drug-induced apoptosis.
In contrast to collagen, fibronectin had no effect on intracellular doxorubicin content, did not up-regulate ABCC1 expression, and did not protect the cells from apoptosis, despite the fact that fibronectin-binding integrins are also expressed in T-ALL cells (Ivanoff et al., 2005). The differential ability of collagen and fibronectin to up-regulate ABCC1 and to promote doxorubicin resistance could be due to their differential ability to activate the ERK/MAPK survival pathway, as it will be discussed later.
Collagen did not up-regulate the expression levels of other doxorubicin transporters, including ABCB1, ABCC2, ABCC6, and ABCG2. In fact, ABCB1, ABCC2, ABCC6, and ABCG2 were not detected in T-ALL cell lines, which is in agreement with previous studies (Martel et al., 1997; van der Heijden et al., 2004; Franco and Cidlowski, 2006; Hammond et al., 2007). Culture of rat hepatocytes in collagen sandwich has been shown to up-regulate ABCB1 (Lee, 2002; Turncliff et al., 2006). Although the role of integrins has not been investigated, these studies support our findings and the notion that ECM–β1 integrin interactions can regulate ABC transporters.
We also found that collagen promotes doxorubicin resistance by activating the prosurvival ERK/MAPK signaling pathway. Our results show that inhibition of ERK reversed collagen-mediated reduction of intracellular doxorubicin content, collagen-mediated up-regulation of ABCC1 expression levels, and collagen-mediated inhibition of doxorubicin-induced apoptosis. The role of ERK in collagen-induced up-regulation of ABCC1 in T-ALL cell lines is further supported by the fact that fibronectin, which we previously showed to be unable to activate the ERK/MAPK pathway in Jurkat cells (Gendron et al., 2003; Chetoui et al., 2006), also failed to up-regulate ABCC1 expression levels. The mechanism by which ERK up-regulates ABCC1 expression levels is unclear. One possibility is that ERK activates certain transcription factors that can up-regulate ABCC1 gene transcription. ERK can activate Ap-1, Notch1, and Sp-1 (Merchant et al., 1999; Xu et al., 2006; Goh et al., 2009), and these transcription factors have been associated with ABCC1 expression (Kurz et al., 2001; Muredda et al., 2003; Cho et al., 2011). Further studies are needed to determine how collagen/β1 integrin signaling up-regulates ABCC1 expression.
Our results also show that actin polymerization is essential for collagen-induced up-regulation of ABCC1 expression and activity. This could be explained by the fact that actin polymerization is involved in collagen-induced ERK activation in Jurkat cells (Bijian et al., 2007; unpublished data), and ERK, as shown here, is important for the up-regulation of ABCC1 expression levels. In addition, and as previously reported (Hummel et al., 2011), actin polymerization could also regulate the function of ABCC1 by stabilizing its localization at the plasma membrane, suggesting that collagen can also enhance ABCC1 activity through inducing actin polymerization.
Collagen production in the tumor tissue can interfere with intratumoral uptake of chemotherapeutic agents (Netti et al., 2000; Loeffler et al., 2006). Thus, in addition, our study indicates that collagen can also favor drug resistance via up-regulation of ABC transporters. It is interesting to note that hypoxic conditions were previously reported to modulate the expression and activation of ABC transporters such as ABCC1 (Thews et al., 2009) and ABCB1 (Lotz et al., 2007; Thews et al., 2011) in cancer cells. Moreover, hypoxia also induces collagen production (Corpechot et al., 2002; Grobe et al., 2007), suggesting that the effect of hypoxia on ABC transporters could be due to collagen signaling. Together these studies point to the importance of collagen–tumor cell interactions in the establishment of the drug resistance phenotype.
In summary, our study provides the first demonstration that collagen/β1 integrin contributes to doxorubicin resistance via up-regulation of ABCC1. Further understanding of the mechanisms by which collagen up-regulates ABCC1 is likely to lead to novel therapeutic strategies for preventing the appearance of the drug resistance phenotype.
MATERIALS AND METHODS
Reagents and antibodies
RPMI 1640, fetal bovine serum (FBS), penicillin–streptomycin, and l-glutamine were purchased from Wisent (St. Bruno, Canada). Collagen type I (collagen), doxorubicin, cytochalasin B, and mouse anti-FLAG antibody (M2) were from Sigma-Aldrich (St. Louis, MO). Human plasma fibronectin and the anti-ABCC1 monoclonal antibody (mAb; clone QCRL-1) used in Western blots were purchased from Millipore (Billerica, MA). The MEK-1 inhibitor (U0126), the ABCC1 inhibitor (MK571), and calcein-AM were from Calbiochem (San Diego, CA). Reversan was from Cedarlane (Burlington, Canada). The blocking anti-β1 integrin (clone 4B4) and the appropriate immunoglobulin G (IgG) isotypic control antibodies were purchased from Beckman Coulter (Brea, CA). The fluorescein isothiocyanate (FITC)–conjugated anti-ABCC1 (clone QCRL-3) and the appropriate IgG isotype control antibodies used in flow cytometry analysis were from BD PharMingen (San Diego, CA). Phalloidin–Alexa Fluor 594 was from Molecular Probes (Invitrogen, Burlington, Canada).
Cell culture
The human T-ALL cell lines Jurkat (E6.1) and HSB2 were obtained from the American Type Culture Collection (Manhasset, VA) and were maintained in RPMI 1640 medium supplemented with 10% FBS, 2 mmol/l glutamine, and 100 U/ml penicillin and streptomycin.
Flow cytometry and fluorescence microscopy analysis of intracellular drug content
Jurkat and HSB2 cells in RPMI medium containing 2.5% FBS were activated with or without collagen or fibronectin (100 μg/ml) or with vehicle for 4 h and then treated with doxorubicin (250 ng/ml) or with calcein-AM (1–5 nM) for different periods of time at 37°C in the dark. The cells were then washed three times with phosphate-buffered saline (PBS), and doxorubicin or calcein-AM intracellular content was analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Diego, CA) using the FL-2 and the FL-1 settings, respectively. The data are expressed as percentage of positive cells times mean fluorescence intensity (MFI) as previously described (Han et al., 2009; El Azreq et al., 2011).
For microscopy analysis, the cells were washed twice with PBS, fixed with 1% paraformaldehyde (30 min at 4°C in the dark), and washed with PBS. The samples were then resuspended in PBS (20 μl) and carefully spread over glass slides. Gel mount reagent (Biomeda, Foster City, CA) was used to mount the cover slips, which were viewed under a fluorescence microscope (BX-51; Olympus, Tokyo, Japan) using the 40× objective connected to a digital camera (CoolSNAP; Photometrics, Tucson, AZ). The general shape of the cells was observed by phase contrast, and the doxorubicin content was revealed using a red excitation fluorescence filter.
Reverse transcriptase-PCR analysis
Total RNA was extracted with TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). First-strand cDNA was prepared from 1 μg of total RNA using the Thermoscript reverse transcriptase-PCR system from Invitrogen. ABCC1, ABCC2, ABCC6, ABCB1, ABCG2, and β-actin transcripts were amplified by PCR using specific primers (Table 1). The amplification for each gene was in the linear curve. PCRs were done with 1 U Taq polymerase in a total volume of 50 μl, and amplifications were carried out in a Peltier Thermal Cycler from MJ Research (St. Bruno, Canada). ABC transporters and β-actin transcripts were amplified for 39 and 29 cycles, respectively, as follows: ABCC1: 94°C for 30 s, 57°C for 1 min, 72°C for 2 min; ABCC2: 94°C for 30 s, 56°C for 1 min, 72°C for 2 min; ABCC6: 94°C for 30 s, 55°C for 1 min, 72°C for 2 min; ABCB1: 94°C for 30 s, 55°C for 1 min, 72°C for 2 min; ABCG2: 94°C for 30 s, 59°C for 1 min, 72°C for 2 min; and actin: 94°C for 30 s, 58°C for 35 s, 72°C for 2 min. Amplified products were separated on a 2% agarose gel and were detected by RedSafe and UV illumination in a GelDoc molecular imager (Bio-Rad, Hercules, CA).
TABLE 1:
Primer sequences used for reverse transcriptase-PCR analysis of ABCC1, ABCC2, ABCC6, ABCB1, ABCG2, and actin expression.
| Protein | Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Reference |
|---|---|---|---|---|
| MRP1 | ABCC1 | AGGTCAAGCTTTCCGTGTACTG | GGACTTTCGTGTGCTCCTGA | Hammond et al. (2007) |
| MRP2 | ABCC2 | CTGCGGTGGATCTAGAGACAGA | TGCCGCACTCTATAATCTTCCC | Hammond et al. (2007) |
| MRP6 | ABCC6 | AGTTCTGTTTGTTACCCACCAGTT | ACCCTTGTCTTGTGACTTCTTCTGT | Hammond et al. (2007) |
| MDR1 | ABCB1 | CCCATCATTGCAATAGCAGG | GTTCAAACTTCTGCTCCTGA | Noonan et al. (1990) |
| BCRP | ABCG2 | GGGTTCTCTTCTTCCTGACGACC | TGGTTGTGAGATTGACCAACAGACC | Scharenberg et al. (2002) |
| Actin | AGCCATGCCAATCTCATCTTGT | ACGGCTGCTTCCAGCTCCTC | Gendron et al. (2005) |
ABCC1 expression by flow cytometry
Jurkat and HSB2 leukemic T-cell lines in RPMI medium containing 2.5% FBS were activated with or without collagen or fibronectin (100 μg/ml) for 4 h before their treatment with 250 ng/ml doxorubicin for different periods of time. The cells were washed with PBS, permeabilized with a CytoFix/CytoPerm kit (BD Biosciences), and stained with FITC-conjugated anti-ABCC1 mAb (20 μl/test; clone QCRL-3), which recognizes an epitope located in the intracellular domain of ABCC1, or with isotypic control antibody. Stained cells were then washed and analyzed by flow cytometry (FACSCalibur; BD Biosciences). The data are expressed as percentage of positive cells times MFI as previously described (Han et al., 2009; El Azreq et al., 2011).
Immunoblot analysis
After stimulation, the cells were washed in cold PBS and lysed in RIPA buffer containing protease and phosphatase inhibitors. Cell lysates were subjected to SDS–PAGE and analyzed by immunoblot using specific anti-ABCC1 mAb (clone QCRL-1). The blots were stripped and reprobed with control anti–β-actin antibody to ensure equal loading. In all experiments, immunoblots were visualized using a horseradish peroxidase–conjugated secondary antibody, followed by enhanced chemiluminescence detection (Pierce, Rockford, IL).
ABCC1 siRNA and silencing
Jurkat and HSB2 T-cell lines were transfected using the Nucleofector (program C-016; Amaxa Biosystems, Cologne, Germany). Cells (5 × 106) were transfected with ON-TARGETplus smart pool (200 nM) containing a mix of four siRNAs directed against ABCC1 (L-007308-00-0005; Dharmacon, Lafayette, CO; Beedholm-Ebsen et al., 2010) or with a control nonsilencing siRNA (Dharmacon). After nucleofection, cells were immediately transferred to prewarmed complete medium and were used in subsequent experiments at 24 h posttransfection. The efficiency of ABCC1 silencing was assessed by Western blot and flow cytometry analysis of ABCC1 expression levels.
Plasmid transfection
The empty plasmid (pcDNA) and the plasmid encoding the dominant-negative form of MEK-1 (DN-MEK-1) were previously used in our studies (Gendron et al., 2003; Aoudjit et al., 2004). Jurkat or HSB2 cells (5 × 106) were transfected with 7 μg of total plasmid DNA using the Nucleofector (program C-016). The cells were immediately allowed to recover in prewarmed RPMI containing 10% FBS and were used in subsequent experiments at 24 h posttransfection. DN-MEK1 expression was monitored by Western blot using an anti-FLAG antibody.
Determination of apoptosis
Jurkat and HSB2 T-cells in RPMI medium containing 2.5% FBS were activated or not with collagen (100 μg/ml) or with vehicle for 4 h. The cells were then treated with doxorubicin (250 ng/ml) for 16–48 h. Apoptosis was determined using the annexin V-FITC detection kit from BD PharMingen. The cells were washed in PBS and incubated in 500 μl of 1× buffer containing 5 μl of annexin V-FITC for 15 min at room temperature in the dark according to the manufacturer's protocol. The cells were then analyzed by flow cytometry using the FL-1 setting (FACSCalibur). Apoptotic cells were identified as being annexin V positive.
Determination of intracellular F-actin content
Jurkat and HSB2 T-cell lines in RPMI medium containing 2.5% FBS were incubated or not with cytochalasin B (10 μM) for 1 h. The cells were then activated or not with collagen for 4 h. The cells were washed three times with PBS, permeabilized with a CytoFix/CytoPerm kit, and stained with Alexa Fluor 594–conjugated phalloidin (1 μM). Stained cells were washed and then analyzed by flow cytometry (FACSCalibur). Data are expressed as percentage of positive cells times MFI.
Statistical analysis
Statistical analysis was performed by the Student's t test. Results with p < 0.05 are considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (MOP-98005) to F.A. D.N. holds a Pierre J. Durand Ph.D. Scholarship from the Faculté de Médecine de l'Université Laval. We thank Jean Sévigny (Université Laval) for providing the CaCo2 cell line.
Abbreviations used:
- ABC
ATP-binding cassette
- CB
cytochalasin B
- ECM
extracellular matrix
- MDR
multidrug resistance
- MRP-1
multidrug resistance–related protein 1
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-02-0132) on July 11, 2012.
REFERENCES
- Abdul-Ghani R, Serra V, Gyorffy B, Jurchott K, Solf A, Dietel M, Schafer R. The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 2006;25:1743–1752. doi: 10.1038/sj.onc.1209201. [DOI] [PubMed] [Google Scholar]
- An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert Opin Drug Metab Toxicol. 2009;5:1529–1542. doi: 10.1517/17425250903228834. [DOI] [PubMed] [Google Scholar]
- Angelini A, Ciofani G, Baccante G, Di Febbo C, Carmine DI, Cuccurullo F, Porreca E. Modulatory effects of heparin on cellular accumulation and cytotoxicity of doxorubicin in MRP1-overexpressing HL60/doxo cells. Anticancer Res. 2007;27:351–355. [PubMed] [Google Scholar]
- Aoudjit F, Guo W, Gagnon-Houde JV, Castaigne JG, Alcaide-Loridan C, Charron D, Al-Daccak R. HLA-DR signaling inhibits Fas-mediated apoptosis in A375 melanoma cells. Exp Cell Res. 2004;299:79–90. doi: 10.1016/j.yexcr.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Engagement of the alpha2beta1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood. 2000;95:2044–2051. [PubMed] [Google Scholar]
- Beedholm-Ebsen R, van de Wetering K, Hardlei T, Nexo E, Borst P, Moestrup SK. Identification of multidrug resistance protein 1 (MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. Blood. 2010;115:1632–1639. doi: 10.1182/blood-2009-07-232587. [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62:6172–6177. [PubMed] [Google Scholar]
- Bijian K, Zhang L, Shen SH. Collagen-mediated survival signaling is modulated by CD45 in Jurkat T cells. Mol Immunol. 2007;44:3682–3690. doi: 10.1016/j.molimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, Cuvillier O. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20:95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- Burkhart CA, et al. Small-molecule multidrug resistance-associated protein 1 inhibitor Reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma. Cancer Res. 2009;69:6573–6580. doi: 10.1158/0008-5472.CAN-09-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BM, Wong JG, Rao A, Hemler ME. T cell receptor-dependent, antigen-specific stimulation of a murine T cell clone induces a transient, VLA protein-mediated binding to extracellular matrix. J Immunol. 1991;147:398–404. [PubMed] [Google Scholar]
- Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–3245. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetoui N, Gendron S, Chamoux E, Aoudjit F. Collagen type I-mediated activation of ERK/MAP kinase is dependent on Ras, Raf-1 and protein phosphatase 2A in Jurkat T cells. Mol Immunol. 2006;43:1687–1693. doi: 10.1016/j.molimm.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Cho S, Lu M, He X, Ee PL, Bhat U, Schneider E, Miele L, Beck WT. Notch1 regulates the expression of the multidrug resistance gene ABCC1/MRP1 in cultured cancer cells. Proc Natl Acad Sci USA. 2011;108:20778–20783. doi: 10.1073/pnas.1019452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Consoli U, et al. Multidrug resistance mechanisms in chronic lymphocytic leukaemia. Br J Haematol. 2002;116:774–780. doi: 10.1046/j.0007-1048.2002.03344.x. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- El Azreq MA, Garceau V, Bourgoin SG. Cytohesin-1 regulates fMLF-mediated activation and functions of the beta2 integrin Mac-1 in human neutrophils. J Leukoc Biol. 2011;89:823–836. doi: 10.1189/jlb.0410222. [DOI] [PubMed] [Google Scholar]
- Fodale V, Pierobon M, Liotta L, Petricoin E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J. 2011;17:89–95. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- Gendron S, Couture J, Aoudjit F. Integrin alpha2beta1 inhibits Fas-mediated apoptosis in T lymphocytes by protein phosphatase 2A-dependent activation of the MAPK/ERK pathway. J Biol Chem. 2003;278:48633–48643. doi: 10.1074/jbc.M305169200. [DOI] [PubMed] [Google Scholar]
- Gendron S, Couture J, Aoudjit F. Collagen type I signaling reduces the expression and the function of human receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes. Eur J Immunol. 2005;35:3673–3682. doi: 10.1002/eji.200535065. [DOI] [PubMed] [Google Scholar]
- Goh F, et al. Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling. Immunology. 2009;127:326–337. doi: 10.1111/j.1365-2567.2008.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 2007;113:357–364. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- Gutmann H, Fricker G, Torok M, Michael S, Beglinger C, Drewe J. Evidence for different ABC-transporters in Caco-2 cells modulating drug uptake. Pharm Res. 1999;16:402–407. doi: 10.1023/a:1018825819249. [DOI] [PubMed] [Google Scholar]
- Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol. 2005;70:695–699. doi: 10.1016/j.bcp.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282:14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- Han L, Wierenga AT, Rozenveld-Geugien M, van de Lande K, Vellenga E, Schuringa JJ. Single-cell STAT5 signal transduction profiling in normal and leukemic stem and progenitor cell populations reveals highly distinct cytokine responses. PLoS One. 2009;4:e7989. doi: 10.1371/journal.pone.0007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hummel I, Klappe K, Ercan C, Kok JW. Multidrug resistance-related protein 1 (MRP1) function and localization depend on cortical actin. Mol Pharmacol. 2011;79:229–240. doi: 10.1124/mol.110.069013. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, et al. Adult T-cell leukemia cells over-express the multidrug-resistance-protein (MRP) and lung-resistance-protein (LRP) genes. Int J Cancer. 1999;82:599–604. doi: 10.1002/(sici)1097-0215(19990812)82:4<599::aid-ijc21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ivanoff J, Talme T, Sundqvist KG. The role of chemokines and extracellular matrix components in the migration of T lymphocytes into three-dimensional substrata. Immunology. 2005;114:53–62. doi: 10.1111/j.1365-2567.2004.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz EU, Cole SP, Deeley RG. Identification of DNA-protein interactions in the 5′ flanking and 5′ untranslated regions of the human multidrug resistance protein (MRP1) gene: evaluation of a putative antioxidant response element/AP-1 binding site. Biochem Biophys Res Commun. 2001;285:981–990. doi: 10.1006/bbrc.2001.5262. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Pande P, Patton WF. Sensitive and specific fluorescent probes for functional analysis of the three major types of mammalian ABC transporters. PLoS One. 2011;6:e22429. doi: 10.1371/journal.pone.0022429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH. Differential regulation of P-glycoprotein genes in primary rat hepatocytes by collagen sandwich and drugs. J Cell Biochem. 2002;86:12–20. doi: 10.1002/jcb.10207. [DOI] [PubMed] [Google Scholar]
- Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells. 2004;17:188–202. [PubMed] [Google Scholar]
- Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007;17:239–244. [PubMed] [Google Scholar]
- Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- Martel J, Payet MD, Dupuis G. The MDR1 (P-glycoprotein) and MRP (P-190) transporters do not play a major role in the intrinsic multiple drug resistance of Jurkat T lymphocytes. Leuk Res. 1997;21:1077–1086. doi: 10.1016/s0145-2126(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- Merchant JL, Du M, Todisco A. Sp1 phosphorylation by Erk 2 stimulates DNA binding. Biochem Biophys Res Commun. 1999;254:454–461. doi: 10.1006/bbrc.1998.9964. [DOI] [PubMed] [Google Scholar]
- Muredda M, Nunoya K, Burtch-Wright RA, Kurz EU, Cole SP, Deeley RG. Cloning and characterization of the murine and rat mrp1 promoter regions. Mol Pharmacol. 2003;64:1259–1269. doi: 10.1124/mol.64.5.1259. [DOI] [PubMed] [Google Scholar]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- Noonan KE, et al. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci USA. 1990;87:7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain S, Lepelley P, Preudhomme C, Cambier N, Cornillon J, Wattel E, Cosson A, Fenaux P. Expression of the multidrug resistance-associated protein in myelodysplastic syndromes. Br J Haematol. 2000;110:591–598. doi: 10.1046/j.1365-2141.2000.02247.x. [DOI] [PubMed] [Google Scholar]
- Prime-Chapman HM, Fearn RA, Cooper AE, Moore V, Hirst BH. Differential multidrug resistance-associated protein 1 through 6 isoform expression and function in human intestinal epithelial Caco-2 cells. J Pharmacol Exp Ther. 2004;311:476–484. doi: 10.1124/jpet.104.068775. [DOI] [PubMed] [Google Scholar]
- Roos PH, Bolt HM. Cytochrome P450 interactions in human cancers: new aspects considering CYP1B1. Expert Opin Drug Metab Toxicol. 2005;1:187–202. doi: 10.1517/17425255.1.2.187. [DOI] [PubMed] [Google Scholar]
- Ruzza P, Rosato A, Rossi CR, Floreani M, Quintieri L. Glutathione transferases as targets for cancer therapy. Anticancer Agents Med Chem. 2009;9:763–777. doi: 10.2174/187152009789056895. [DOI] [PubMed] [Google Scholar]
- Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–178. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Gillet JP, Sauerbrey A, Gruhn B, Dawczynski K, Bertholet V, de Longueville F, Zintl F, Remacle J, Efferth T. ABCA3 as a possible cause of drug resistance in childhood acute myeloid leukemia. Clin Cancer Res. 2006;12:4357–4363. doi: 10.1158/1078-0432.CCR-05-2587. [DOI] [PubMed] [Google Scholar]
- Streuli CH. Integrins and cell-fate determination. J Cell Sci. 2009;122:171–177. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thews O, Nowak M, Sauvant C, Gekle M. Hypoxia-induced extracellular acidosis increases p-glycoprotein activity and chemoresistance in tumors in vivo via p38 signaling pathway. Adv Exp Med Biol. 2011;701:115–122. doi: 10.1007/978-1-4419-7756-4_16. [DOI] [PubMed] [Google Scholar]
- Thews O, Sauvant C, Gekle M. Impact of the metabolic tumor microenvironment on activity and expression of the multidrug resistance-related protein 1 (MRP1) Acta Physiol. 2009;195(Suppl 669):O123. [Google Scholar]
- Turncliff RZ, Tian X, Brouwer KL. Effect of culture conditions on the expression and function of Bsep, Mrp2, and Mdr1a/b in sandwich-cultured rat hepatocytes. Biochem Pharmacol. 2006;71:1520–1529. doi: 10.1016/j.bcp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Van de Walle GR, Vanhoorelbeke K, Majer Z, Illyes E, Baert J, Pareyn I, Deckmyn H. Two functional active conformations of the integrin α2β1, depending on activation condition and cell type. J Biol Chem. 2005;280:36873–36882. doi: 10.1074/jbc.M508148200. [DOI] [PubMed] [Google Scholar]
- van der Heijden J, de Jong MC, Dijkmans BA, Lems WF, Oerlemans R, Kathmann I, Schalkwijk CG, Scheffer GL, Scheper RJ, Jansen G. Development of sulfasalazine resistance in human T cells induces expression of the multidrug resistance transporter ABCG2 (BCRP) and augmented production of TNFalpha. Ann Rheum Dis. 2004;63:138–143. doi: 10.1136/ard.2002.005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, Keum YS, Nair S, Kong AN. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.