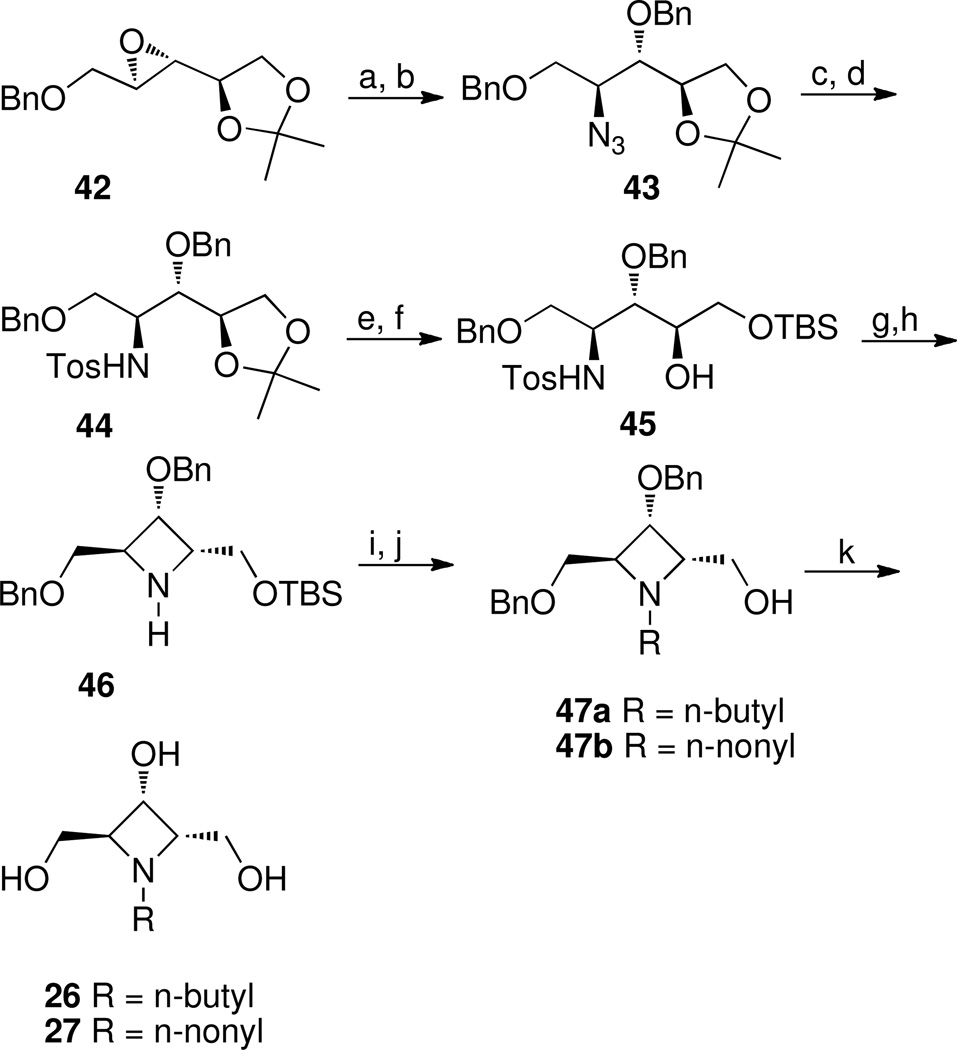
Scheme 9a.
aReagents and conditions: (a) NaN3, NH4Cl, 2-methoxyethanol, water 9:1, reflux; (b) NaH, benzyl bromide, TBAI, THF, rt, 1 h, 70% over two steps; (c) LiAlH4, THF; (d) tosyl chloride, triethylamine, CH2Cl2, rt, 88% over two steps (e) 2N HCl: methanol, 40 °C; (f) TBSCl, triethylamine, DMAP, CH2Cl2, 87% over two steps; (g) PPhe3, DIAD, CH2Cl2, rt; (h) Na, naphthalene, DME, −60 °C, 55% over two steps; (i) aldehyde (butyraldehyde or nonyl aldehyde), sodium triacetoxyborohydride, ClCH2CH2Cl, rt; (j) TBAF, THF, rt 72% and 76% respectively over two steps; (k) PdCl2, H2, methanol 68% and 74% respectively.