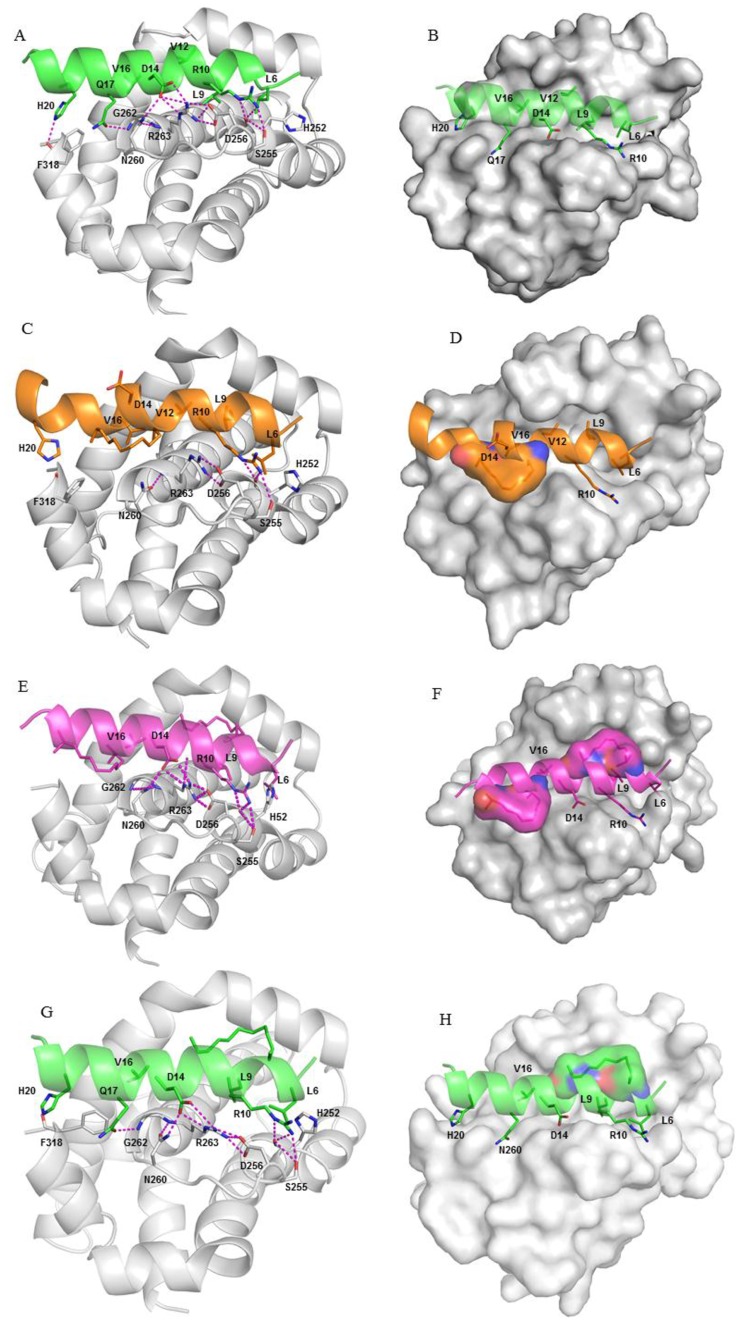
Figure 4. BH3wt bound to MCL-1 (shown in grey).
(A) Asp14 of the peptide interacts with Arg263 and Asn260 of MCL-1; Arg263 also interacts with Asp256; Arg10 hbonds with Ser255; His20 sidechain hbonds with the backbone of Phe318; Gln17 sidechain hbonds with the backbone of Gly262 (B) The hydrophobic groups Leu6, Leu9, and Val16 are buried in the hydrophobic binding groove on the surface of MCL-1 (shown in surface); BHC bound to MCL-1 (shown in grey) (C) The location of the staple forces it to point into the MCL-1 surface creating a steric clash, thus creating a strain on the backbone of the BH3C peptide and its helicity. The loss of key hbond networks result in decreased contributions from Arg10 and Asp14 when compared with BH3wt peptide (shown in cartoon), (D) MCL-1 bound to BH3C peptide (shown in surface); BH3H bound to MCL-1 (shown in grey) (E) Double stapling improves the packing of the stapled regions and also maintains the helical content (in cartoon) (F) The hydrophobic groups Leu6, Leu9, and Val16 are buried in the hydrophobic binding groove on the surface of MCL-1 (shown in surface); BH3K bound to MCL-1 (shown in grey) (G) This staple interacts with the hydrophobic patch on the MCL-1 surface but also enables Gln17 to stabilize the system by hbonding to the backbone of Gly262 (shown in cartoon) (H) The hydrophobic groups Leu6, Leu9, and Val16 are buried in the hydrophobic binding groove on the surface of MCL-1 (shown in surface).