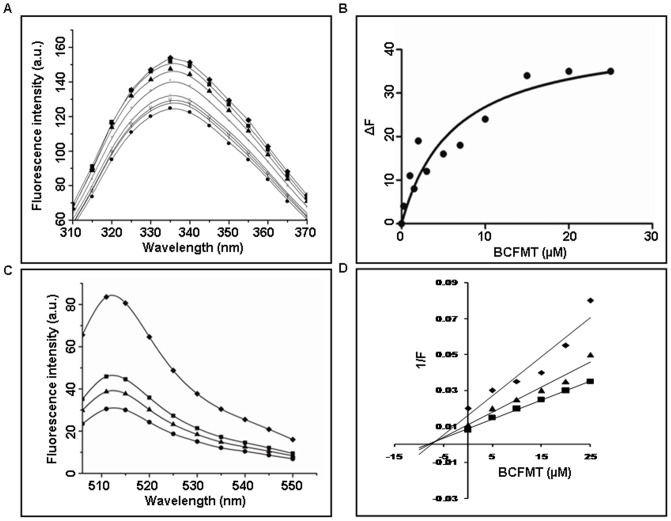
Figure 3. BCFMT bound to purified tubulin and inhibited the binding of BODIPY FL-vinblastine to tubulin.
(A) The effects of BCFMT on the tryptophan fluorescence spectra of tubulin are shown. Spectra were monitored in the absence (♦) and presence of 0.25 (▪), 0.5 (▴), 1 (×), 2 (−), 5 (○), 7 (l) and 10 (•) µM BCFMT. (B) The change in the fluorescence intensity of tubulin (ΔF) was plotted against concentration of BCFMT. The dissociation constant (Kd) for BCFMT binding to tubulin was estimated using an equation described in the methods. Data were the average of four independent experiments. (C) Reduction in the fluorescence intensity of tubulin- BODIPY FL-vinblastine complex in the absence (♦) and presence of 10 (▪), 25 (▴) and 50 (•) µM BCFMT. (D) Tubulin (2 µM) in 25 mM PIPES buffer (pH 6.8) was incubated without and with different concentrations (5, 10, 15, 20, 25 µM) of BCFMT at 25°C for 20 min. Three such different sets were prepared. After 20 min incubation, in one set 2 µM (♦), in the second set 4 µM (▴) and in the third set 6 µM (▪) BODIPY FL-vinblastine was added. Fluorescence of tubulin-BODIPY FL-vinblastine complex was measured and the inhibitory concentration (Ki) was calculated from the modified Dixon plot.