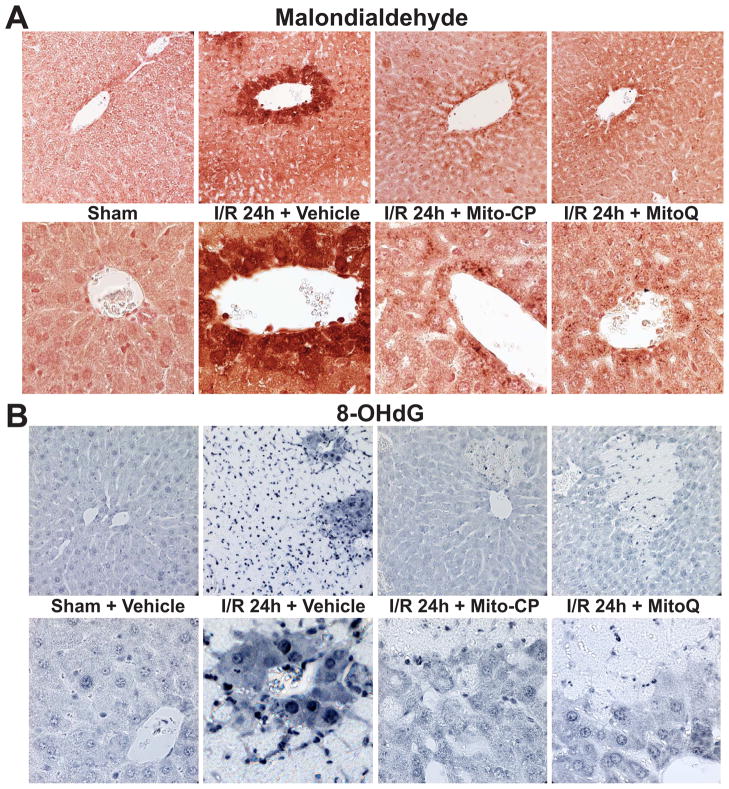
Figure 6. MitoQ and Mito-CP treatment attenuates the I/R-induced increased hepatic malondialdehyde formation and oxidative DNA damage.
Panel A: Malondialdehyde staining (brown; a marker of lipid peroxidation/oxidative stress) of representative liver sections of sham mice treated with vehicle (sham) or MitoQ/Mito-CP (antioxidants in sham mice had no effects, not shown), and mice exposed to 1 hour of ischemia followed by 24 hours of reperfusion (I/R 24h) treated with vehicle or MitoQ/Mito-CP (3 mg/kg i.p.). 24 hours of I/R triggers marked increase in liver malondialdehyde formation, which is predominantly localized to endothelial cells, perivascular hepatocytes, and infiltrating (or attached to the endothelium) inflammatory cells, and these increases are markedly attenuated by pretreatment with MitoQ/Mito-CP. Minimal staining is seen in the livers of control mice exposed to sham surgery. Slides are counterstained by nuclear fast red. Upper row of images depicts 400× magnification, while the lower one 1000× magnification. A similar histological profile was seen in three to five livers/group.
Panel B: 8-OHdG staining (blue; marker of oxidative DNA damage) of representative liver sections of sham mice treated with vehicle (sham) or MitoQ/Mito-CP (antioxidants in sham mice had no effects, not shown) and mice exposed to 1 hour of ischemia followed by 24 hours of reperfusion (I/R 24h) treated with vehicle or MitoQ/Mito-CP. 24 hours of I/R triggers markedly increased 8-OHdG formation in endothelial cells, perivascular hepatocytes and infiltrating inflammatory cells, and these changes are attenuated by pretreatment with MitoQ/Mito-CP. Please note that the necrotic areas are lighter and infiltrated by neutrophils showing intense nuclear staining. Minimal nuclear 8-OHdG staining is seen in the livers of control mice exposed to sham surgery. Upper row of images depicts 400× magnification, while the lower one 1000× magnification. A similar histological profile was seen in three to five livers/group.