Abstract
HF-ACTION was a multicenter, randomized, controlled trial designed to examine the safety and efficacy of aerobic exercise training versus usual care in 2,331 patients with systolic heart failure (HF). In HF-ACTION patients with resting transthoracic echocardiographic (echo) measurements, we examined predictive value of 8 echo-Doppler measurements—left ventricular (LV) diastolic dimension, mass, systolic (ejection fraction) and diastolic function (mitral valve [MV] peak early diastolic-to-peak late diastolic [E/A], peak MV early diastolic velocity-to-tissue Doppler peak early diastolic myocardial velocity [E/E’] ratios, and deceleration time), left atrial (LA) dimension, and mitral regurgitation severity (MR)—for primary endpoint of all-cause death or hospitalization and secondary endpoint of cardiovascular disease (CVD) death or HF hospitalization. We also compared prognostic value of echo variables versus peak oxygen consumption (VO2). MV E/A and E/E’ ratios were more powerful independent predictors of clinical endpoints than was LV ejection fraction (LVEF), but less powerful than peak VO2. In multivariate analyses for predicting primary endpoint, adding E/A ratio to a basic demographic/clinical model increased C-index from 0.61 to 0.62, compared with 0.64 after adding peak VO2. For secondary endpoint, 6 echo variables, but not LVEF or LA dimension, provided independent predictive power over basic model. Addition of E/E’ or E/A to the basic model increased C-index from 0.70 to 0.72 and 0.73, respectively (all p <0.0001). Simultaneously adding E/A and peak VO2 to basic model increased C-index to 0.75 (p <0.0005). No echo variable was significantly related to 0-to-3 month change in exercise peak VO2. In conclusion, addition of echo LV diastolic function variables improves prognostic value of a basic demographic/clinical model for CVD outcomes.
Keywords: Systolic heart failure, echocardiography, exercise training, clinical outcomes
The current analysis examines the prognostic power of baseline Doppler-echocardiography (echo) measures of left ventricular (LV) and left atrial (LA) anatomy, LV systolic and diastolic function, and mitral regurgitation (MR) for overall and cardiovascular disease (CVD)-related outcomes, and 3-month exercise training effect in Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) patients. The major hypothesis was: Increased LV mass, LV internal dimension, LA dimension, and MR severity; decreased LV ejection fraction (LVEF); and decreased LV diastolic function, as measured at baseline by Doppler echo, will: (1) improve the prediction, over a basic model of demographic and clinical variables, of increased all-cause death or all-cause hospitalization (primary endpoint), as well as CVD death or heart failure (HF) hospitalization (secondary endpoints), over a 30-month median follow-up period; and (2) predict a poorer exercise training effect, as measured by baseline-to-3 month change in exercise peak oxygen consumption (VO2), in the exercise training intervention group.
Methods
The design (1), primary outcome (2), and baseline Doppler-echo findings (3) of the HF-ACTION study have been previously reported. Enrollment criteria included an LVEF ≤35%, New York Heart Association (NYHA) clinical class II-IV HF, and sufficient ability to undergo exercise training. Patients were excluded if they were unable to exercise, already exercising regularly, or had experienced a CVD event in the prior 6 weeks. Patients were treated optimally according to current practice guidelines (2). Overall, 2,331 patients were randomly assigned to either participate in 36 sessions of facility-based, followed by home-based, exercise training for the remainder of the trial, in addition to usual care, or receive usual care alone; median follow-up was approximately 2.5 years.
Doppler-echocardiography was performed at baseline using standard methodology; echo recordings were forwarded to a core laboratory for analysis (3,4). Studies were read blinded as to demographic and clinical information by a primary reader and overread by an experienced Level III echocardiographer using a measurement workstation (Digisonics, Inc, Houston, TX). The following echo variables were measured or derived: LV mass, diastolic dimension (LVDD), volumes, and LVEF; LA dimension, peak MV early diastolic (E) velocity, average of septal and lateral myocardial annular tissue velocity (E’), E/E’ ratio, peak early diastolic-to-peak late diastolic (E/A) velocity ratio, early diastolic deceleration time (Dec Time), and MR grade (4–6). MR was graded from apical-view color Doppler echo images, as follows: none, 0; trace, 1; mild, 2; mild-to-moderate, 3; moderate, 4; moderately severe, 5; and severe, 6. LV dimensions, wall thickness, and mass, and LA dimension, were measured from 2-dimensionally derived M-mode echocardiograms. If M-mode echocardiograms were judged suboptimal, linear dimensions were measured from 2-dimensional (2D) images (7). Peak E and A MV pulsed-Doppler velocities were measured at the mitral leaflet tip level during diastole in the apical 4-chamber view. Septal and lateral E’ myocardial velocities were recorded with sample volumes positioned within 1 cm of septal and lateral insertion sites, respectively, of the anterior and posterior mitral leaflets (8). Measures of decreased LV diastolic function included abnormal E/A ratio (<0.75 or >1.5), decreased early diastolic Dec Time, increased E/E’ ratio, and increased LA dimension (8).
Symptom-limited exercise (CPX) testing with gas exchange measurement was completed using commercially-available metabolic carts and motor driven treadmills, employing a modified Naughton protocol in 91% and cycle ergometers in 9% of subjects (9). Exercise test supervisors encouraged patients to exercise to exhaustion. The respiratory exchange ratio was used to confirm satisfactory exercise effort. Peak VO2 was determined in a core laboratory as the highest oxygen consumption normalized to body mass (VO2, mL/kg/min) for a 15- or 20-second interval during last 90 seconds of exercise or first 30 seconds of recovery (9). The independent relationships of baseline demographic and clinical variables to clinical outcomes were assessed using bootstrapped, step-down variable selection. Based partially on this assessment, the following were included in models to determine the independent predictive ability of echo variables for primary or secondary CVD outcomes: age, gender, race, body surface area, geographic region, Kansas City Cardiomyopathy Questionnaire (KCCQ) symptom stability score, blood urea nitrogen (BUN), ventricular conduction, beta blocker dose, and loop diuretic dose.
Univariate and multivariate Cox regression were used to analyze relations of demographic/clinical, echo-Doppler and exercise training (peak VO2) variables to the primary and secondary outcomes. The bootstrap-corrected C-index was used to evaluate predictive ability of multivariate models for both primary and secondary outcomes. In the exercise training group, univariate correlations between echo-Doppler variables and change in peak VO2 between baseline and 3 months of training were examined using linear regression analysis. Kaplan-Meier curves were used to display event rates. Statistical analyses were performed using SAS (version 8.2, SAS Institute Inc, Cary, North Carolina) and R Design Library (version 2.9.2, The R Foundation for Statistical Computing). Statistical significance was set at the two-tailed alpha =.05 level, with no adjustment for multiple comparisons. Unless otherwise indicated, all P values are based on the likelihood ratio chi-square statistic.
Results
Table 1 presents selected demographic, clinical, and echo variables in the overall cohort (n=2,331) and in the subgroup (n=519) for whom complete data were available for the primary endpoint in multivariate models. Most patients in the cohort were men, white, and were in New York Heart Association clinical class II and class III HF. There were no qualitative differences in demographic (age, sex, BMI, and race), exercise, and LVEF variables between the overall cohort and the echo subgroup. The largest source of missing data was related to E’ measurements being available in only 909 patients (see Table 2) because tissue velocity measurements were not routinely recorded at some centers.
Table 1.
Baseline Demographic, Clinical and Echocardiographic Characteristics of Participants as a Function of Echocardiographic Measurement Availability
| Parameter | Overall cohort (n = 2331) | Cohort with complete echo data for primary endpoint (n = 519) |
|---|---|---|
| Age (years) | 59 (51, 68) | 59 (50, 68) |
| Men | 72% | 69% |
| White/Black/Other | 62%, 33%, 5% | 59%, 34%, 7% |
| Body surface area (m2) | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) |
| Blood urea nitrogen (mg/dL) | 20 (15, 28) | 20 (14, 28) |
| Diabetes mellitus | 32% | 32% |
| Left ventricular ejection fraction | 25% (20, 30) | 25% (21, 31) |
| New York Heart Association Class (II, III) | 63%, 36% | 65%, 35% |
| Peak oxygen consumption (ml/kg/min) | 14.4 (11.5, 17.7) | 15.8 (11.8, 17.8) |
| Ventricular conduction | 13%, 17%, 43%, 24%, 4% | 13%, 15%, 47%, 21%, 4% |
Continuous variables are expressed as median (25th percentile, 75th percentile)
Ventricular conduction is categorized as interventricular conduction delay, left bundle branch block, normal, paced rhythm, and right bundle branch block, respectively.
Table 2.
Univariate Predictors of HF-ACTION Primary Endpoint (All-Cause Death or All-Cause Hospitalization)
| Echo Parameters | Sample Size | Hazard Ratio (95% confidence interval) | Chi-Square Value | p-value |
|---|---|---|---|---|
| Left ventricular diastolic dimension (cm) | 1646 | 1.09 (1,04, 1.15) | 12.3 | 0.0005 |
| Left ventricular mass (per 100g) | 1646 | 1.08 (1.04, 1.12) | 13.5 | 0.0002 |
| Left ventricular ejection fraction (per 5%) | 2327 | 0.89 (0.86, 0.92) | 49.7 | <0.0001 |
| Left atrial dimension (cm) | 1646 | 1.30 (1.21, 1.41) | 48.1 | <0.0001 |
| Peak mitral early diastolic-to-peak late diastolic velocity ratio | 1550 | 1.15 (1.08, 1.22) | 19.5 | <0.0001 |
| Early diastolic deceleration time (msec) | 1604 | 0.91 (0.87, 0.95) | 18.6 | <0.0001 |
| Tissue Doppler peak early diastolic myocardial velocity (cm/sec) | 909 | 0.98 (0.96, 1.00) | 6.4 | 0.01 |
| Peak mitral early diastolic velocity-to-tissue Doppler peak early diastolic myocardial velocity ratio | 796 | 1.03 (1.01, 1.04) | 18.7 | <0.0001 |
| Mitral regurgitation grade(grades 0–4 vs. 5–6) | 2135 | 1.53 (1.31, 1.77) | 27.8 | <0.0001 |
| Peak oxygen consumption(ml/kg/min) | 2275 | 0.92 (0.91, 0.93) | 199.0 | <0.0001 |
Abbreviations are as in text.
Table 2 outlines univariate predictors of the primary endpoint (all-cause hospitalization or all-cause death). Among the 2,331 HF-ACTION patients, measurements for LVDD, LV mass, LA dimension, E/A, and Dec Time were available for 1,550–1,646 patients. Tissue Doppler-based parameters—including E’ velocity and E/E’ velocity—were present in only 909 and 796 patients, respectively. Except for E’ velocity (barely significant), all echo variables were highly statistically significant univariate predictors of the primary endpoint; however, peak VO2 was a better predictor than any echo variable.
Table 3 shows C-index and multivariate p-values for the primary endpoint when each echo variable was separately added to the basic multivariate model (which included only 519 patients who had non-missing data for all variables). Only E/A increased (slightly) the C-index of the basic model (from 0.61 to 0.62, p =0.003); nevertheless, E/A and E/E’ had highly significant chi-square p-values. (A significant chi-square p-value can indicate statistical improvement in model fit by inclusion of a variable in the absence of substantive improvement in model discrimination between higher and lower risk patients, denoted by C-index [10].) The other 7 echo variables added little to prediction beyond that achieved by the basic multivariate model plus E/A. Importantly, peak VO2 improved risk discrimination independently of the basic model and echo variables, increasing C-index from 0.62 to 0.64, while echo variables did not improve risk discrimination of the basic model plus peak VO2 with C-index remaining unchanged at 0.64.
Table 3.
Multivariate Models for HF-ACTION Primary Endpoint (n=519 with complete data)
| Multivariate Model | Multivariate Model Chi-Square Value | Multivariate p-value of added predictor(s) beyond the Basic model | C-index |
|---|---|---|---|
| Basic | 57.8 | 0.61 | |
| Basic + Left ventricular diastolic dimension | 58.4 | 0.49 | 0.61 |
| Basic + Left ventricular mass | 58.0 | 0.69 | 0.61 |
| Basic + Left ventricular ejection fraction | 58.1 | 0.60 | 0.61 |
| Basic + Left atrial dimension | 61.1 | 0.07 | 0.61 |
| Basic + Peak mitral early diastolic-to-peak late diastolic velocity ratio | 66.6 | 0.003 | 0.62 |
| Basic + Early diastolic deceleration time | 60.2 | 0.12 | 0.61 |
| Basic + Peak mitral early diastolic velocity-to-tissue Doppler peak early diastolic myocardial velocity ratio | 65.6 | 0.005 | 0.61 |
| Basic + Mitral regurgitation grade | 62.7 | 0.08 | 0.61 |
| Basic + All 8 echo variables | 74.6 | multiple added predictors | 0.62 |
| Basic + Peak oxygen consumption | 92.5 | <0.0001 | 0.64 |
| Basic + Peak oxygen consumption + Peak mitral early diastolic-to-peak late diastolic velocity ratio | 94.8 | 0.13 (E/A) <0.0001 (peak VO2) | 0.64 |
Basic multivariate model for primary endpoint includes beta blocker dose (truncated at 50mg/day), body surface area, BUN, gender, KCCQ symptom stability score, region (U.S. vs. non-U.S.), ventricular conduction.
Table 4 displays the univariate predictors for the secondary combined endpoint (CVD mortality or HF hospitalization). All echo variables, except for E’ velocity, were highly statistically significant predictors of the secondary endpoint. LA dimension, LVEF, MR grade, E/A, and E/E’ were the most important echo predictors of the secondary endpoint, but peak VO2 was even more important. Table 5 shows multivariate p-values and C-indices for the secondary endpoint when each of the 8 echo variables was separately added to the basic multivariate model. The multivariate models included only patients who had data for all variables. E/A and E/E’ were the most statistically significant echo variables; their addition to the basic model resulted in the most substantial increases in C-index (from 0.70 for the basic model to 0.73 and 0.72, respectively). However, peak VO2 was a stronger independent predictor for the secondary endpoint (C-index =0.74) than any echo variable. Moreover, peak VO2 was an independent predictor of outcomes even when all 8 echo variables were included. There was no difference in predictive ability between the basic model plus all 8 echo variables and peak VO2 versus the basic model plus E/A and peak VO2. In the 972 individuals in the exercise training arm with serial measurements, no echo variable was significantly related to baseline-to-3 month change in peak VO2.
Table 4.
Univariate Predictors of HF-ACTION for Secondary Endpoint (Cardiovascular Disease Mortality or Heart Failure Hospitalization)
| Echo Parameters | Sample Size | Hazard Ratio (95% confidence interval) | Chi-Square Value | p-value |
|---|---|---|---|---|
| Left ventricular diastolic dimension (cm) | 1646 | 1.14 (1.06, 1.23) | 12.7 | 0.0004 |
| Left ventricular mass (per 100g) | 1646 | 1.10 (1.04, 1.17) | 10.8 | 0.001 |
| Left ventricular ejection fraction (per 5%) | 2327 | 0.82 ( 0.78, 0.87) | 58.3 | <0.0001 |
| Left atrial dimension (cm) | 1646 | 1.48 (1.33, 1.65) | 49.7 | <0.0001 |
| Peak mitral early diastolic-to-peak late diastolic velocity ratio | 1550 | 1.43 (1.33, 1.54) | 71.2 | <0.0001 |
| Early diastolic deceleration time (per 50msec) | 1604 | 0.83 (0.77, 0.89) | 27.9 | <0.0001 |
| Tissue Doppler peak early diastolic myocardial velocity(cm/sec) | 909 | 0.98 (0.95, 1.01) | 2.24 | 0.13 |
| Peak mitral early diastolic velocity-to-tissue Doppler peak early diastolic myocardial velocity ratio | 796 | 1.23 (1.15, 1.33) | 25.5 | <0.0001 |
| Mitral regurgitation grade(grades 0–4 vs. 5–6) | 2135 | 2.3 (1.9, 2.8) | 61.1 | <0.0001 |
| Peak oxygen consumption(ml/kg/min) | 2275 | 0.86 (0.85, 0.88) | 255.3 | <0.0001 |
Table 5.
Multivariate Models for HF-ACTION Cardiovascular Disease Mortality or Heart Failure Hospitalization Endpoint (n=512 with complete data)
| Multivariate Model | Multivariate Model Chi-Square Value | Multivariate p-value of added predictor(s) beyond the basic model | C-index |
|---|---|---|---|
| Basic | 100.4 | 0.70 | |
| Basic + Left ventricular diastolic dimension | 107.1 | 0.009 | 0.71 |
| Basic + Left ventricular mass | 105.3 | 0.03 | 0.70 |
| Basic + Left ventricular ejection fraction | 103.8 | 0.06 | 0.70 |
| Basic + Left atrial dimension | 101.3 | 0.33 | 0.70 |
| Basic + Peak mitral early diastolic-to-peak late diastolic velocity ratio | 127.4 | <0.0001 | 0.73 |
| Basic + Early diastolic deceleration time | 105.6 | 0.02 | 0.71 |
| Basic + Peak mitral early diastolic velocity-to-tissue Doppler peak early diastolic myocardial velocity ratio | 121.2 | <0.0001 | 0.72 |
| Basic + Mitral regurgitation grade | 111.6 | 0.0008 | 0.71 |
| Basic + All 8 echo variables | 149.5 | multiple added predictors | 0.74 |
| Basic + Peak oxygen consumption | 132.6 | <0.0001 | 0.74 |
| Basic + Peak oxygen consumption + Peak mitral early diastolic-to-peak late diastolic velocity ratio | 146.4 | 0.0002 (E/A) <0.0001 (peak VO2) | 0.75 |
Basic multivariate model for secondary endpoint includes age (truncated at 62 years), body surface area, BUN (truncated at 39mg/dL), gender, KCCQ symptom stability score, loop diuretic dose (truncated at 100mg), race, ventricular conduction.
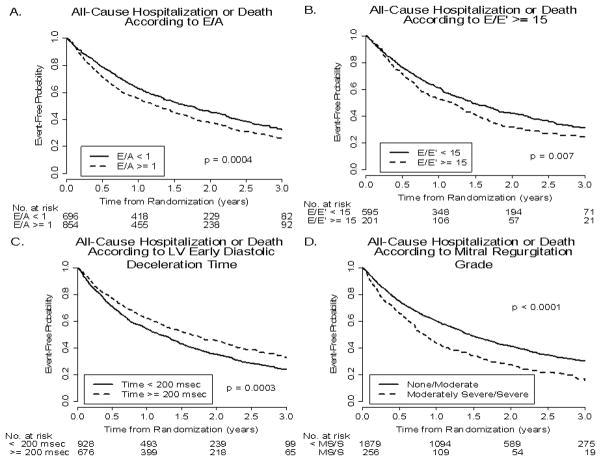
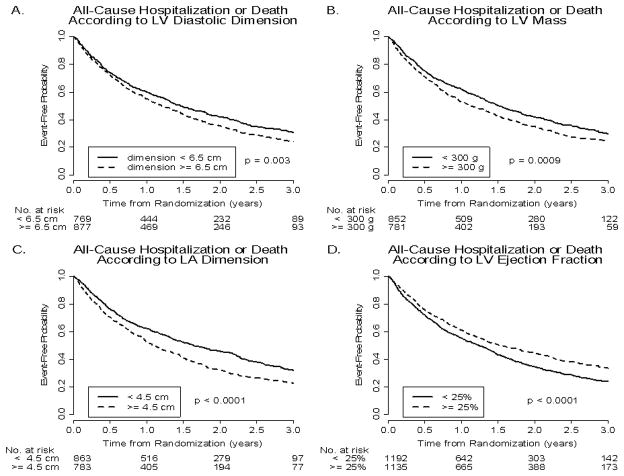
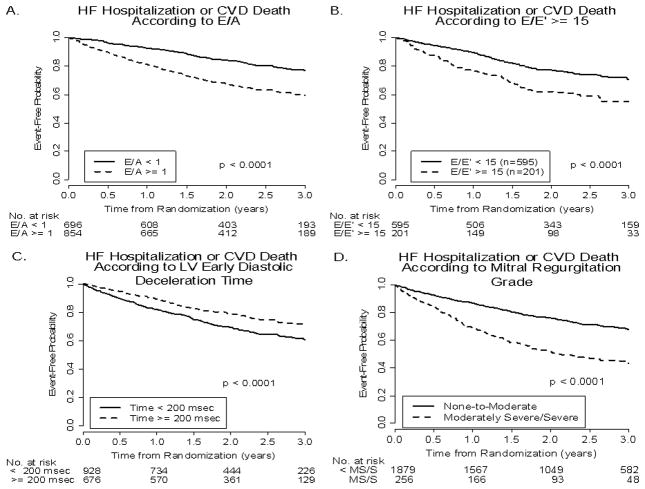
Figures 1 and 2 present Kaplan-Meier curves demonstrating the relationship of the event-free probability for primary outcome versus time from randomization in patients with each echo variable above and below a defined clinically-relevant cutpoint. Note that (Figure 1) event-free probability for primary outcome was higher with an E/A < versus ≥1.0, E/E’ < versus ≥15, Dec Time < versus ≥200 msec, and MR grades of < moderately severe (0–4) versus moderately severe or severe (5, 6). Figure 2 presents similar relationships in patients with LVDD < versus ≥6.5 cm, LV mass < versus ≥300 g, LA dimension < versus ≥4.5 cm, and LVEF < versus ≥25%. Event-free probabilities for the primary outcome were significantly higher in those with smaller LVDD, smaller LV mass, smaller LA dimension, and higher LVEF. Relations similar to those for the primary outcome were present between all 8 echo variables and the secondary outcome. Significant differences were present between the 2 curves for each echo variable, representing event-free probabilities for patients with echo measurements above and below the defined clinically-relevant cutpoint. Figure 3 presents relationships (all significant) between the secondary outcome and E/A, E/E’, LV Dec Time and MR grade. Visual differences between the 2 event-free curves were greatest for MR grade.
Figure 1.
Kaplan-Meier curves for the event-free probabilities for all-cause hospitalization or death (primary outcome) as a function of E/A ratio <1.0 versus ≥1.0 (panel A); E/E’ <15 versus ≥15 (panel B); Dec Time <200 versus ≥200 msec (panel C); and MR, none-to-moderate versus moderately severe or severe (panel D).
Figure 2.
Kaplan-Meier curves for the event-free probabilities for all-cause hospitalization or death (primary outcome) as a function of LVDD <6.5 vs ≥6.5 cm (Panel A), LV mass <300 vs ≥300 g (Panel B), LA dimension <4.5 vs ≥4.5 cm (Panel C), and LVEF <25% vs ≥25% (Panel D).
Figure 3.
Kaplan-Meier curves for the event-free probabilities for HF hospitalization or CVD death (secondary outcome) as a function of E/A, E/E’, Dec Time, and MR. Format is the same as in Figure 1.
Discussion
We examined predictive value for all-cause death or all-cause hospitalization (primary endpoint) and CVD death or HF hospitalization (secondary endpoint) of Doppler-echo measures of LV and LA anatomy, and LV systolic and diastolic function, in the HF-ACTION cohort. For the primary endpoint, peak VO2 was a more powerful univariate and multivariate predictor than were echo variables when added to a basic demographic and clinical model. Moreover, peak VO2 improved risk prediction independently of the basic model and the echo variables, while the echo variables did not improve the predictive ability of the basic model once peak VO2 was included. Similarly, for the secondary endpoint, peak VO2 was a more important univariate predictor than the echo variables. Adding peak VO2 was equivalent as an independent multivariate predictor of the secondary endpoint to adding all 8 echo variables to a basic model. E/A was the most important single echo predictor for both primary and secondary endpoints. For the secondary endpoint, including E/A in the basic model with peak VO2 improved C-index modestly. However, LVEF was not an independent predictor beyond the basic multivariate model for primary or secondary endpoints.
We believe the HF-ACTION cohort is the largest to measure both echo variables and aerobic capacity using CPX testing in patients with systolic HF. Our study extends previous work by suggesting that in patients with systolic HF, a combination of commonly recorded resting echo variables may add modest prognostic value to peak VO2; however, peak VO2 is a stronger predictor of adverse outcomes than any individual echo variable. Kaplan-Meier event rate analysis showed significantly higher rates of overall and CVD/HF hospitalization and mortality in the groups with: (1) greater LVDD, LV mass, LA dimension, E/A and E/E’ ratios, and MR severity; and (2) lesser LVEF and Dec Time. This study also extends our previous findings (4) that baseline Doppler-echo measures of LV diastolic function—including E/A and E/E’—were modest, but better independent predictors in this cohort of baseline aerobic exercise capacity (peak VO2) and ventilatory efficiency (VE/VCO2 slope) than was LVEF.
Measures of LV systolic function, LV mass, and LV diastolic function/filling—e.g., E/A, E/E’, and Dec Time—have been shown to predict CVD events in patients with systolic HF. In the SOLVD Registry and Trials, LV mass ≥298 g and LA dimension ≥4.17 cm were associated with increased risk of death and CVD hospitalization in 1,172 patients with LV dysfunction. A protective effect of LVEF >35%—i.e., better outcomes—was noted only in patients with LV mass ≥298 g (11). In 207 consecutive patients with dilated cardiomyopathy, indexed LA size was the best predictor of death in patients >70 years old, whereas a “restrictive mitral flow pattern” (Dec Time <140 ms) was independently associated with cardiac death or HF hospitalization (12). In smaller studies of ischemic and non-ischemic cardiomyopathy patients, with LVEF cutpoints ranging from <50% to <35% and E/E’ cutpoints ranging from 13.5 to 16, E/E’ ratio was a good predictor of cardiac death or HF rehospitalization and of a combined endpoint including death, heart transplantation, and HF hospitalization (8,13–16). Dokainish, et al., reported that E/E’ and pre-discharge brain natriuretic peptide blood levels were incremental predictors of cardiac death or rehospitalization for HF (17). Our study extends previous work by demonstrating that, in our cohort, echo-Doppler E/A and E/E’ ratios and MR grade are stronger predictors of HF hospitalization or CVD mortality than are LV mass, LVEF, and LA dimension.
There are a number of likely reasons why the resting echo-Doppler variables studied were not better predictors—e.g., as compared to peak VO2—of the primary or secondary outcomes. Tests that examine cardiopulmonary function during stress—e.g., exercise CPX tests—often have more robust diagnostic and prognostic capabilities than those examining only resting function. Furthermore, echo-Doppler variables do not assess non-cardiac HF components—e.g., abnormalities of skeletal muscle or peripheral vasculature—or multiple comorbidities that may drive many events in HF patients (17,18). Of importance, age alone is a strong predictor of overall and CVD-related outcomes; after adjustment for age in a multivariate model, echo-Doppler variables have substantially less prognostic power.
Several limitations of the current study are apparent. First, echo variables were not available in many patients. M-mode echo variables—e.g., LVDD, LV mass, and LA dimension—and pulsed Doppler E/A and Dec Time—were available in 2/3rd, whereas tissue Doppler-based variables—E’ velocity and E/E’—were available in only 1/3rd of the cohort. In approximately 1/3rd, 2D-derived M-mode measurements of LVDD, LV mass, and LA dimension could not be reliably performed, thereby limiting the usefulness of echo in these patients and others outside the study in whom these measurements cannot be reliably made. Nonetheless, as reported previously (3), our findings should be generalizable to the entire cohort because there were no meaningful differences in demographic or clinical variables between subgroups in whom all echo variables were available and the entire cohort. Second, there are well-known limitations in using Doppler-echo measurements to evaluate LV systolic and diastolic function. Potential difficulties include LV foreshortening, inadequate visualization of LV endocardium, and mathematical over-simplifications in 2D models used to estimate three-dimensional LV volumes, mass, and EF. In patients with severe HF, E/E’ ratio has been reported unreliable in predicting intracardiac filling pressures—especially in patients with large LV volumes (19). E/E’ may reflect either a “restrictive” filling pattern or “pseudonormalization” in patients with high filling pressures (20). Factors including loading conditions and regional contractility may modify the E/E’. Currently, there is no single perfect Doppler-echo measurement of diastolic dysfunction. Nonetheless, the HF-ACTION core echo laboratory has previously reported measurements for inter-reader variability of 2 ± 1% (mean ± standard deviation) for E velocity and 5 ± 2–3% for Dec Time and E’ velocity (21). Third, since a follow-up echo was not performed, we cannot comment on 3-month changes in echo variables potentially associated with either baseline-to-3 month change in peak VO2 or primary or secondary endpoints. Fourth, because patients included in this study were preselected on the basis of their ability to participate in the exercise training protocol, the results of this study cannot be extrapolated to all patients with advanced systolic HF. Finally, plasma natriuretic peptides, strong predictors of outcomes in systolic HF (22), were not routinely measured, preventing assessment of the independent prognostic power of echo variables in this context.
Acknowledgments
Funding Source
This study was supported by funding from the National Institutes of Health.
The authors thank Danielle Rivas for her careful preparation of this manuscript.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA Piña IL; HF-ACTION Trial Investigators. Heart Failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F Piña IL; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardin JM, Leifer ES, Fleg JL, Whellan D, Kokkinos P, Leblanc MH, Wolfel E, Kitzman DW HF-ACTION Investigators. Relationship of Doppler-echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: the HF-ACTION study. Am Heart J. 2009;158:S45–S52. doi: 10.1016/j.ahj.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multi-center investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 5.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quiñones M, Schiller NB, Stein JH, Weissman NJ American Society of Echocardiography. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Bensimhon DR, Leifer ES, Ellis SJ, Fleg JL, Keteyian SJ, Pina IL, Kitzman DW, McKelvie RS, Kraus WE, Forman DE, Kao AJ, Whellan DJ, O’Connor CM, Russel SD HF-ACTION Trial Investigators. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Am J Cardiol. 2008;102:712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 11.Quinones MA, Greenberg BH, Kopelen HA, Kiolpillai C, Limacher MC, Shindler DM, Shelton BJ, Weiner DH SOLVD Investigators. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. J Am Coll Cardiol. 2000;35:1237–1244. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 12.Dini FL, Cortigiani L, Baldini U, Boni A, Nuti R, Barsotti L, Micheli G. Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. Am J Cardiol. 2002;89:518–523. doi: 10.1016/s0002-9149(01)02290-1. [DOI] [PubMed] [Google Scholar]
- 13.Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M, Fogarty A, Morehead AJ, Starling RC, Young JB, Thomas JD, Lauer MS, Klein AL. Usefulness of tissue Doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–262. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 14.Bruch C, Klem I, Breithardt G, Wichter T, Gradaus R. Diagnostic usefulness and prognostic implications of the mitral E/E’ ratio in patients with heart failure and severe secondary mitral regurgitation. Am J Cardiol. 2007;100:860–865. doi: 10.1016/j.amjcard.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Oki T, Yamada H, Tanaka H, Ishimoto T, Wakatsuki T, Tabata T, Ito S. Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J Am Soc Echocardiogr. 2003;16:333–339. doi: 10.1016/s0894-7317(02)74537-9. [DOI] [PubMed] [Google Scholar]
- 16.Dokainish H, Zoghbi WA, Lakkis NM, Ambriz E, Patel R, Quinones MA, Nagueh SF. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol. 2005;45:1223–1226. doi: 10.1016/j.jacc.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Kitzman DW. Exercise training in heart failure with preserved ejection fraction: beyond proof-of-concept. J Am Coll Cardiol. 2011;58:1792–1794. doi: 10.1016/j.jacc.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW. Outcomes in patients with heart failure with preserved ejection fraction: it is more than the heart. J Am Coll Cardiol. 2012;59:1006–1007. doi: 10.1016/j.jacc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70. doi: 10.1161/CIRCULATIONAHA.108.779223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumesnil JG, Paulin C, Pibarot P, Coulombe D, Arsenault M. Mitral annulus velocities by Doppler tissue imaging: practical implications with regard to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15:1226–1231. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 21.Khan S, Bess RL, Rosman HS, Nordstrom CK, Cohen GI, Gardin JM. Which echocardiographic Doppler left ventricular diastolic function measurements are most feasible in the clinical echocardiographic laboratory? Am J Cardiol. 2004;94:1099–1101. doi: 10.1016/j.amjcard.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 22.Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]