Table 2. Trifunctional Catalysis Screening.
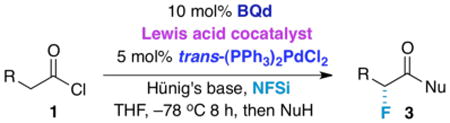
| ||||
|---|---|---|---|---|
|
| ||||
| entrya | third catalyst, (equiv.) | R | NuH | % yield |
| 1 | none | CH2Ph | MeOH | <4 |
| 2 | LiPF6-THF, (0.1) | CH2Ph | MeOH | 21 |
| 3 | LiCI04,(1) | CH2Ph | MeOH | trace |
| 4 | LiCI04, (0.1) | CH2Ph | MeOH | 21 |
| 5 | La(OTf)3,(0.1) | CH2Ph | MeOH | 8 |
| 6 | Sm(OTf)3, (0.1) | CH2Ph | MeOH | 5 |
| 7 | Yb(OTf)3, (0.1) | CH2Ph | MeOH | 3 |
| 8 | LiCI04, (0.1)b | CH2Ph | MeOH | trace |
| 9 | (PPh3)2Pd(CI04)2, (0.05)b | Ph | MeOH | trace |
| 10 | none | CH2Ph | PhNH2 | 4 |
| 11 | none | CH2Ph | PhNH2 | 22c |
| 12 | LiCI04, (0.1) | CH2Ph | PhNH2 | 39c |
| 13 | LiCI04, (0.1) | CH2Ph | PhNH2 | 44d |
| 14 | none | i-Pr | PhNH2 | 15 |
| 15 | LiCI04, (0.1) | i -Pr | PhNH2 | 50 |
| 16 | LiCI04, (0.1) | i -Pr | PhNH2 | 73c |
Reaction conditions: 1 equiv. NFSi, 1 equiv. acid chloride, 0.1 equiv. BQd, 0.05 equiv. frans-(PPh3)2PdCI2, 1.1 equiv. Hünig's base, THF, −78 °C, followed by nucleophilic quench at −78 °C after 8 h.
No trans-(PPh3)2PdCI2 catalyst.
Slow addition of Hünig's base over 12 h.
Slow addition of Hünig's base over 24.
