Abstract
Malignant perivascular epithelioid cell tumor (PEComa) is a rare tumor composed of hybrid tumor cells characterized by immunoreactivity for both melanocytic and smooth muscle markers. This paper describes the uncommon esophageal location of an 8 cm PEComa in a 75-year-old Caucasian man who was presented with ingravescent dysphagia. Although PEComas arising within the gastrointestinal tract are exceptional findings, clinicians should not exclude this class of tumors in the diagnostic investigation of a bulky lesion of the esophageal wall.
1. Introduction
Originally described by Liebow and Castleman in 1971 [1], perivascular epithelioid cell tumor (PEComa; also referred to as clear cell “sugar” tumor) is a category of mesenchymal tumors of relatively recent acquisition in the World Health Organization (WHO) classification of tumors of soft tissue and bone [2].
Histologically, PEComas are composed by a hybrid tumor cell which is characterized by immunoreactivity for both melanocytic (HMB45 and/or Melan-A) and smooth muscle (α-smooth muscle actin and/or desmin) markers [3–8].
In the English literature, about hundred cases (in almost every body site) have been reported; they often occur in middle-aged patients, with a female predominance (female-to-male ratio nearly to 7 : 1) [2–4]. In the PEComa family of tumors some other nosological entities have been included: angiomyolipoma, clear cell “sugar” tumor of the lung, lymphangioleiomyomatosis, clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres, and unusual clear cell tumors of the pancreas, rectum, abdominal serosa, uterus, vulva, thigh, and heart [2].
Data on PEComas arising within the gastrointestinal tract remain limited to isolate case reports [9–22]. This is the first description, to the best of our knowledge, of a case of malignant PEComa of the esophagus.
2. Case Report
2.1. Patient History
A 75-year-old Caucasian man was referred to the Department of General Surgery at the University of Padua Hospital for ingravescent dysphagia. The patient had no medical and no family history of gastroesophageal malignancies. The physical examination showed no abnormalities. Hematological and chemical studies, including tumor markers, gave normal results. A dominant polypoid esophageal mass with distal erosion, measuring 8.0 cm, was documented at EGDS (between 31 and 39 cm from the incisors). Brushing cytology was consistent with poorly differentiated small cell neoplasm and tumor cells were negative for both cytokeratins (MNF116) and chromogranin in immunohistochemical analysis performed on the cell block specimen.
A subsequent total body CT scan confirmed the bulky lesion extending from the carina to the cardial level. Moreover, also numerous hypodense small round lesions involving the liver (extending to all the eight liver segments), and thoracoabdominal lymph nodes (pericardial, lesser curve, and celiac groups) were detected. Also a PET-CT was performed, confirming the CT data (Figure 1).
Figure 1.
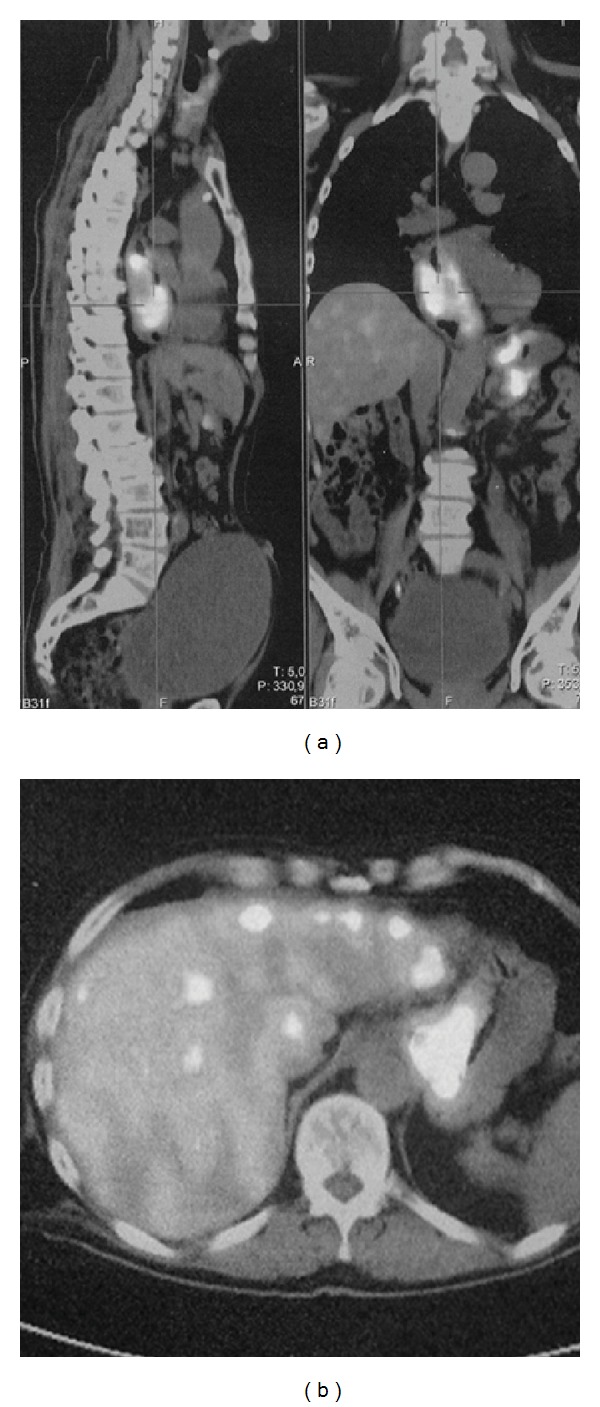
PET-TC scan showing the bulky esophageal mass (a) and the secondary diffuse liver involvement (b).
The patient underwent exploratory laparotomy, which revealed (additionally to the neoplastic liver involvement) diffuse encasing of the diaphragmatic peritoneum and of the omentum by finely scattered nodules alternating with thin, gray-white tumor rinds showing hemorrhagic variegations on the cut surface. Multiple tumor paracardial nodules were biopsied and a percutaneous transperitoneal jejunostomy for delivery of enteral nutrition was placed.
No further therapy was administered. The patient died 3 months after the initial diagnosis for a devastating course of the neoplastic disease and progressive physical deterioration. No postmortem examination was allowed.
2.2. Pathological Findings
Histological evaluation (H&E) on the tumor biopsy samples obtained from the paracardial nodules showed a diffused proliferation of polygonal/spindle cells, characterized by a round to oval nucleus (Figures 2(a)-2(b)) with nospecific growth pattern. The cell cytoplasm was variably abundant, clear to granular. High vascular density areas were observed; tumor cells were steadily separated from capillary walls by eosinophilic bands (Figure 2(a)). Necrosis was not found and tumor exhibited three mitotic figures ×50 high power fields (HPF).
Figure 2.
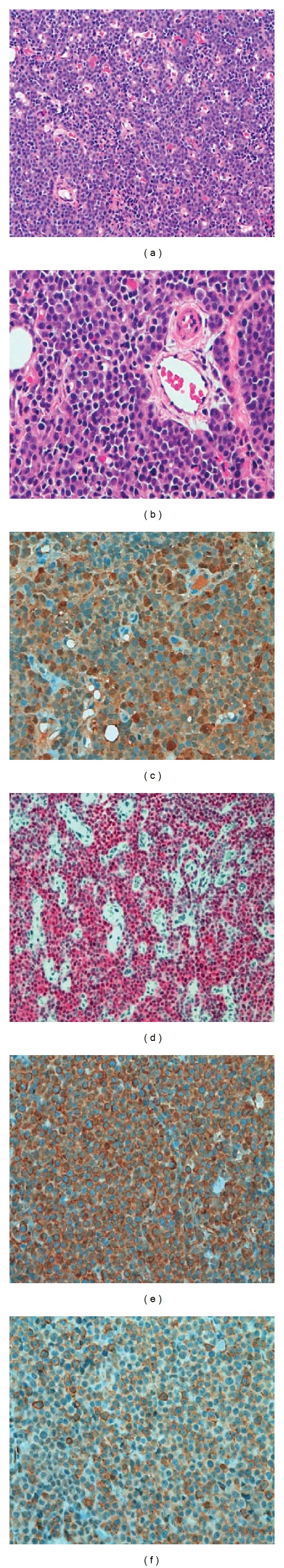
Histological and immunohistochemical features observed in the present case. (a-b) Histological evaluation of hematoxylin and eosin (H&E) stained sections showed a diffused uniform proliferation of polygonal cells, showing a round to oval nucleus. Eosinophilic cell cytoplasm was variably abundant, clear to granular. (c–f) Immunohistochemically, the tumor cells were positive for S100 (c), Mitf (d), vimentin (e), and smooth muscle actin (f). (Original magnifications ×20 and ×40.)
Immunohistochemistry (IHC) was performed on the Ventana automated system (Ventana Benchmark XT system; Touchstone, AZ USA) on 5 μm thick sections obtained from formalin-fixed, paraffin-embedded tissue. The applied antibodies and working dilutions are listed in Table 1. Sections were lightly counterstained with hematoxylin and appropriate positive/negative controls were run concurrently. The immunoreactions were semiquantitatively scored by applying a conventional 4-tiered scale: 0 = no immunoreactions; 1 = positive immunoreactions in 33% (or fewer) of the tumor cells; 2 = positive immunoreactions in 34–65% of cells; 3 = positive immunoreactions in more than 65%. The results of immunohistochemistry are summarized in Table 1 and in Figures 2(c)–2(f). The tumor cells showed strong, diffuse positive cytoplasmic staining to HMB45, S100, vimentin and strong nuclear reactivity to microphthalmia transcription factor (Mitf). Cancer cells were also positive for HHF35, whereas were negative for desmin, CD45 (LCA), CD99, chromogranin, and cytokeratins.
Table 1.
Immunohistochemical profile of the tumor.
| Antigen | Clone and source | Dilution | Score values |
|---|---|---|---|
| Melanosome | HMB45 (Dako, Glostrup, Denmark) | Prediluted | ++− |
| S100 | polyclonal (Ventana, Tucson, AZ, USA) | Prediluted | ++− |
| Vimentin | V9 (BioGenex, San Ramon, CA, USA) | 1 : 100 | ++− |
| Mitf | D5 (Dako) | 1 : 100 | ++− |
| Smooth muscle actin | HHF35 (Dako) | 1 : 800 | +−− |
| Desmin | D33 (Dako) | 1 : 50 | — |
| High molecular weight cytokeratins | 34betaE12 (Ventana) | Prediluted | — |
| Low molecular weight cytokeratins | CAM 5.2 (Becton-Dickinson, Mountainview, CA, USA) | 1 : 10 | — |
| Intermediate/low-molecular-weight cytokeratins | MNF116 (Dako) | 1 : 50 | — |
| CD45 antigen | RP2/18 (Ventana) | Prediluted | — |
| CD99 antigen | 12E7 (Dako) | Prediluted | — |
| Chromogranin | DAK A3 (Dako) | 1 : 100 | — |
| bcl-2 oncoprotein | 124 (Dako) | 1 : 50 | — |
| CD20 antigen | L26 (Ventana) | Prediluted | — |
| CD3 antigen | 2GV6 (Ventana) | Prediluted | — |
| CD138 antigen | B-A38 (Ventana) | Prediluted | — |
| Kappa chains | polyclonal (Ventana) | Prediluted | — |
| Lambda chains | polyclonal (Ventana) | Prediluted | — |
| PDGFRA | C-20 (Dako) | 1 : 100 | — |
3. Discussion
PEComa is a rare mesenchymal neoplasia belonging to a heterogeneous family of tumors, including the pulmonary “sugar” tumor and lymphangiomyomatosis and, in the abdominal cavity, the renal angiomyolipoma and the clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres [2, 23]. Moreover, this group of tumors has been associated to genetic alterations of the tuberous sclerosis complex (TSC) [2–4].
Data on PEComas arising within the gastrointestinal tract remain limited to isolate case reports and 21 cases have been described so far, including 1 in the stomach, 3 in the small bowel, 12 in the colon, 1 in the appendix, and 4 in the rectum [9–22].
By the histological point of view, PEComas are composed by the proliferation of perivascular epithelioid cells (PECs). PECs are hybrid tumor cells characterized by immunoreactivity for both melanocytic and smooth muscle markers, such as HMB45, HMSA-1, MelanA/Mart1, microphthalmia transcription factor (Mitf), actin, and, less commonly, desmin; the immunoreactivity for vimentin is usually inconspicuous [3–5]. The tumors show a perivascular location, with radial arrangement around the vascular lumen of epithelioid/spindled cells, with clear to lightly granular eosinophilic cytoplasm. The pathological findings in our case were consistent with those of PEComa and immunohistochemical data further confirmed the histological diagnosis.
Several neoplasms should be considered in the differential diagnosis of this entity. In fact, in addition to epithelioid smooth muscle tumors (i.e., epithelioid leiomyosarcoma and epithelioid leiomyoma), the other important differential diagnosis of PEComa includes malignant melanoma. PEComas can be differentiated from malignant melanoma based on a negative history for melanoma, visceral location of tumor, perivascular accentuation of tumor cells, immunoreactivity for myoid markers (i.e., smooth muscle actin, muscle-specific actin, and desmin), and absence of the t(12:22) translocation [2]. Another important finding is the S100 positivity observed in melanoma; however, up to 11% of PEComas (as in our case) express S100 as well [23]. According to these data, tumors with strong and diffuse melanocytic marker positivity, and actin immunoreactivity should be designated as PEComas based on morphology, immunophenotype, and clinical history.
Even not well established, criteria for malignancy have been proposed. WHO guidelines suggest that PEComas should be regarded as malignant when they display infiltrative growth, marked hypercellularity, nuclear enlargement, hypercromasia, high mitotic activity, atypical mitotic figures, and coagulative necrosis [2]. More recently it has been reported that tumor size over 5 cm, with infiltrative growth pattern, high nuclear grade, necrosis and mitotic activity over 1/50 HPF, is significantly associated with aggressive clinical behaviour of PEComas of soft tissue and gynaecologic origin [3, 10, 22].
In conclusion, we presented, to the best of our knowledge, the first description of a case of malignant tumor which fulfilled all the morphological, immunohistochemical, and clinical criteria for a final diagnosis of malignant PEComa of the esophagus. Accurate recognition of this entity is essential because of potential misdiagnosis as other malignant tumors, especially malignant melanoma. Although these tumors are exceptional findings, they should not be excluded in the diagnostic investigation of a neoplastic lesion of the esophageal wall.
Consent
Written informed consent was obtained from the patient for publication of this paper and any accompanying images.
Conflict of Interests
There is no conflict of interests to be declared.
References
- 1.Liebow AA, Castleman B. Benign clear cell (“sugar”) tumors of the lung. Yale Journal of Biology and Medicine. 1971;43(4):213–222. [PMC free article] [PubMed] [Google Scholar]
- 2.Folpe AL. Neoplasms with perivascular epithelioid cell differentiation (PEComas) In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 221–222. [Google Scholar]
- 3.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Archiv. 2008;452(2):119–132. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornick JL, Fletcher CDM. PEComa: what do we know so far? Histopathology. 2006;48(1):75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 5.Armah HB, Parwani AV. Perivascular epithelioid cell tumor. Archives of Pathology and Laboratory Medicine. 2009;133(4):648–654. doi: 10.5858/133.4.648. [DOI] [PubMed] [Google Scholar]
- 6.Bonetti F, Pea M, Martignoni G, et al. PEC and sugar. American Journal of Surgical Pathology. 1992;16(3):307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Pea M, Martignoni G, Zamboni G, Bonetti F. Perivascular epithelioid cell. The American Journal of Surgical Pathology. 1996;20(9):1149–1153. doi: 10.1097/00000478-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Salviato T, Altavilla G, Busatto G, Pizzolitto S, Falconieri G. Diffuse intra-abdominal clear cell myomelanocytic tumor: report of an unusual presentation of “PEComatosis” simulating peritoneal mesothelioma. Annals of Diagnostic Pathology. 2006;10(6):352–356. doi: 10.1016/j.anndiagpath.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Mitteldorf CATDS, Birolini D, Da Camara-Lopes LH. A perivascular epithelioid cell tumor of the stomach: an unsuspected diagnosis. World Journal of Gastroenterology. 2010;16(4):522–525. doi: 10.3748/wjg.v16.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SJ, Han DK, Baek HJ, et al. Perivascular epithelioid cell tumor (PEComa) of the ascending colon: the implication of IFN-α2b treatment. Korean Journal of Pediatrics. 2010;53(11):975–978. doi: 10.3345/kjp.2010.53.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman HJ, Webber DL. Perivascular epithelioid cell neoplasm of the colon. World Journal of Gastrointestinal Oncology. 2010;2:205–208. doi: 10.4251/wjgo.v2.i4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross E, Vernea F, Weintraub M, Koplewitz BZ. Perivascular epithelioid cell tumor of the ascending colon mesentery in a child: case report and review of the literature. Journal of Pediatric Surgery. 2010;45(4):830–833. doi: 10.1016/j.jpedsurg.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Shi HY, Wei LX, Sun L, Guo AT. Clinicopathologic analysis of 4 perivascular epithelioid cell tumors (PEComas) of the gastrointestinal tract. International Journal of Surgical Pathology. 2010;18(4):243–247. doi: 10.1177/1066896908330481. [DOI] [PubMed] [Google Scholar]
- 14.Bonetti F, Martignoni G, Colato C, et al. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Modern Pathology. 2001;14(6):563–568. doi: 10.1038/modpathol.3880351. [DOI] [PubMed] [Google Scholar]
- 15.Baek JH, Moon GC, Dong HJ, Jae HO. Perivascular epithelioid cell tumor (PEComa) in the transverse colon of an adolescent: a case report. Tumori. 2007;93(1):106–108. doi: 10.1177/030089160709300120. [DOI] [PubMed] [Google Scholar]
- 16.Birkhaeuser F, Ackermann C, Flueckiger T, et al. First description of a PEComa (perivascular epithelioid cell tumor) of the colon: report of a case and review of the literature. Diseases of the Colon and Rectum. 2004;47(10):1734–1737. doi: 10.1007/s10350-004-0637-5. [DOI] [PubMed] [Google Scholar]
- 17.Evert M, Wardelmann E, Nestler G, Schulz HU, Roessner A, Röcken C. Abdominopelvic perivascular epitheiioid cell sarcoma (malignant PEComa) mimicking gastrointestinal stromal tumour of the rectum. Histopathology. 2005;46(1):115–117. doi: 10.1111/j.1365-2559.2005.01991.x. [DOI] [PubMed] [Google Scholar]
- 18.Genevay M, Mc Kee T, Zimmer G, Cathomas G, Guillou L. Digestive PEComas: a solution when the diagnosis fails to “Fit”. Annals of Diagnostic Pathology. 2004;8(6):367–372. doi: 10.1053/j.anndiagpath.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Mhanna T, Ranchere-Vince D, Hervieu V, Tardieu D, Scoazec JY, Partensky C. Clear cell myomelanocytic tumor (PEComa) of the duodenum in a child with a history of neuroblastoma. Archives of Pathology and Laboratory Medicine. 2005;129(11):1484–1486. doi: 10.5858/2005-129-1484-CCMTPO. [DOI] [PubMed] [Google Scholar]
- 20.Prasad ML, Keating JP, Heng Teoh H, et al. Pleomorphic angiomyolipoma of digestive tract: a heretofore unrecognized entity. International Journal of Surgical Pathology. 2000;8(1):67–72. doi: 10.1177/106689690000800112. [DOI] [PubMed] [Google Scholar]
- 21.Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Modern Pathology. 2001;14(6):615–622. doi: 10.1038/modpathol.3880360. [DOI] [PubMed] [Google Scholar]
- 22.Yanai H, Matsuura H, Sonobe H, Shiozaki S, Kawabata K. Perivascular epithelioid cell tumor of the jejunum. Pathology Research and Practice. 2003;199(1):47–50. doi: 10.1078/0344-0338-00353. [DOI] [PubMed] [Google Scholar]
- 23.Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. American Journal of Surgical Pathology. 2000;24(9):1239–1246. doi: 10.1097/00000478-200009000-00007. [DOI] [PubMed] [Google Scholar]


