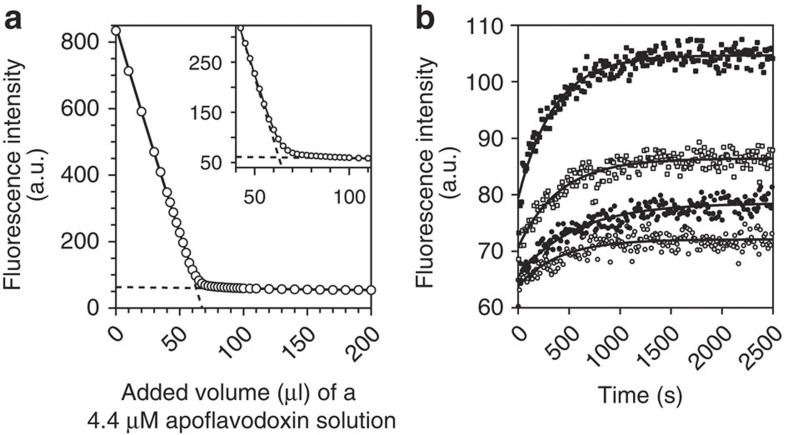
Figure 1. Equilibrium constant for dissociation of FMN from flavodoxin and corresponding rate constant.
(a) Determination of KD using the quenching of FMN fluorescence on binding of this cofactor to apoprotein at 25 °C. A 188-nM FMN solution is titrated with aliquots of a 4.4-μM apoflavodoxin solution. One unit of fluorescence corresponds to 0.22 nM FMN. Equation (S8) of Supplementary Methods is fitted to the resulting fluorescence intensity data and KD is determined to be (3.51±0.20)·10−10 M. The two linear components of this equation are shown as dashed lines. The inset highlights the curved part of the titration data. (b) On dilution of flavodoxin in buffer at 25 °C, FMN is released and fluorescence increases accordingly (fluorescence emission of FMN is followed at 525 nm, while excitation occurred at 450 nm). One unit of fluorescence corresponds to 5.0 nM FMN. After dilution of 5 (open circles), 10 (closed circles), 20 (open squares) and 50 (closed squares) μl of 362 μM flavodoxin in 2,000 μl buffer, respectively, reestablishment of the equilibrium between apo- and holoprotein proceeds exponentially as function of time. Equation (S11) of Supplementary Methods is globally fitted to the four relaxation traces and shows that koff is (1.05±0.04)·10−6 s−1 and KD is (3.82±0.12)·10−12 M.