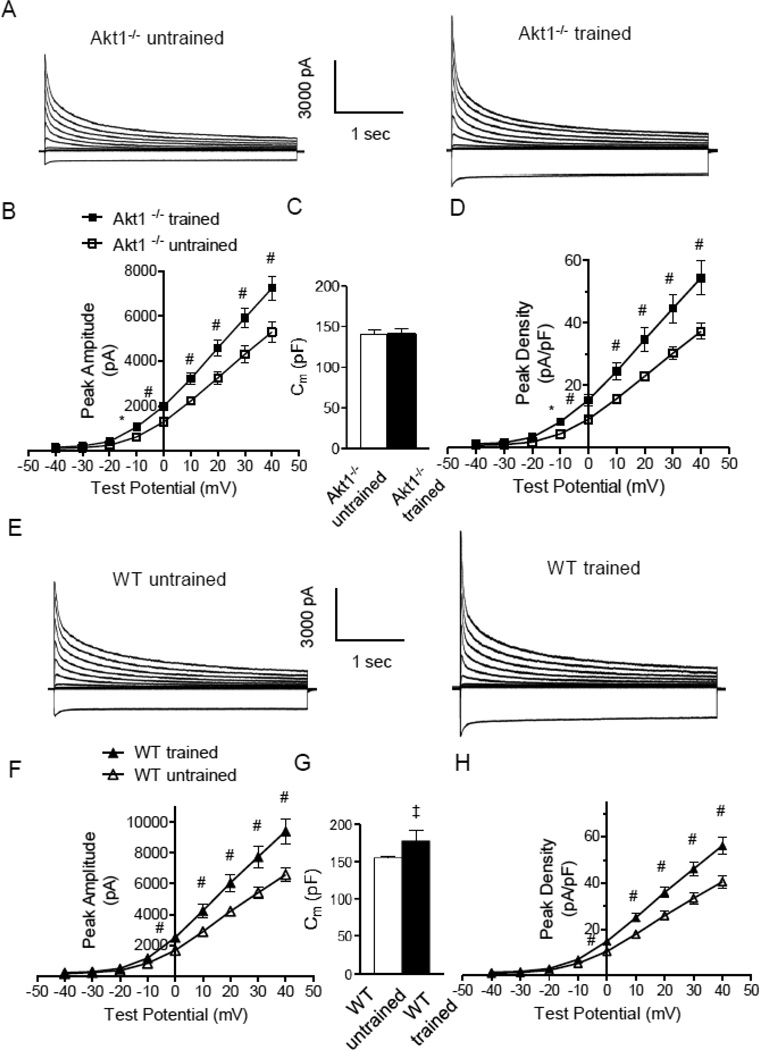
Figure 2. Repolarizing K+ current amplitudes and densities were increased in LV myocytes isolated from swim-trained Akt1−/− animals.
(A) Representative whole-cell K+ currents, recorded from myocytes isolated from the LV apex of swim-trained and untrained C57Bl/6 Akt1−/− mice, are illustrated. Currents were evoked in response to (4.5 s) voltage steps to test potentials between −120 and +40 mV from a holding potential (HP) of −70 mV. (B) The mean ± SEM amplitudes of the peak outward K+ currents were significantly (#P<0.01,*P<0.001) higher in LV apex myocytes from swim-trained, compared with untrained Akt1−/− animals. (C) Mean ± SEM whole-cell membrane capacitances (Cm) were similar in LV myocytes isolated from swim-trained and untrained Akt1−/− animals, consistent with the absence of hypertrophic growth in response to exercise training. (D) Normalizing current amplitudes to cell size (Cm) revealed that mean ± SEM IK,peak densities were also significantly higher in LV apex myocytes from swim-trained, compared with untrained, Akt1−/− animals. (E) Representative whole-cell K+ currents, recorded from myocytes isolated from the LV apex of swim-trained and untrained WT (C57Bl/6) mice, are illustrated. (F) The mean ± SEM amplitudes of the peak outward K+ currents (IK,peak) were significantly (#P<0.01) higher in LV apex myocytes from swim-trained, compared with untrained, WT (C57Bl/6) animals. (G) Mean ± SEM whole-cell Cm were significantly (‡P<0.05) higher in LV myocytes isolated from swim-trained, compared to untrained, WT (C57Bl/6) animals, consistent with the development of hypertrophic growth in response to chronic swim training. (H) Normalizing current amplitudes to whole-cell membrane capacitance revealed that mean ± SEM IK,peak densities were also significantly higher in LV apex myocytes from swim-trained, compared with untrained, WT animals.