Abstract
Deficits in visual processing are well established in schizophrenia. However, there is conflicting evidence about whether these deficits start before the formation of percepts because visual processing studies in schizophrenia have typically examined the processing of consciously registered stimuli. In this study, we used nonconscious color priming to evaluate the very early visual processing stages in schizophrenia. Nonconscious and conscious color priming was assessed in 148 schizophrenia patients and 54 healthy control subjects. In both conditions, subjects identified the color of a ring preceded by a disk (prime) in the same color (congruent) or a different color (incongruent). The ring rendered the disk invisible in the nonconscious condition (SOA of 62.5 ms) or did not mask the disk (SOA of 200 ms) in the conscious condition. Schizophrenia patients exhibited a color priming effect (longer reaction times in the incongruent vs. congruent trials) that was similar to healthy controls in both the nonconscious and conscious priming conditions. Healthy controls had a significantly larger priming effect in the nonconscious vs. conscious condition, but patients did not show a significant difference in priming effects between the two conditions. Our results indicate that schizophrenia patients do not have deficits at the nonconscious, pre-perceptual stages of visual processing, suggesting that the feed forward sweep of information processing (from retina to V1) might be intact in schizophrenia. These results imply that the well-documented visual processing deficits in this illness likely occur at later, percept-dependent stages of processing.
Keywords: schizophrenia, visual processing, color priming, nonconscious, feed forward
Introduction
Patients with schizophrenia exhibit a wide range of cognitive and perceptual impairments, including deficits in basic visual processing, such as visual motion perception (e.g., Chen et al., 1999) and backward masking (e.g., Butler et al., 2002; Green et al., 1994; Rund et al., 2004). These early-stage sensory processing deficits have consequences for the downstream processing of higher-level social cognitive tasks and functioning (Brittain et al., 2010; Norton et al., 2009; Rassovsky et al., 2011; Sergi et al., 2002).
Studies of visual backward masking in schizophrenia have found highly replicable deficits at both early and late perceptual stages of processing (Green et al., 2011a). What remains unclear is how early in the visual processing stream these deficits commence. Studies have been inconsistent regarding whether deficits exist at the earliest processing stages. There is some evidence of deficits occurring as early as input at the retina. For example, a study using electroretinography (Balogh et al., 2008) reported that schizophrenia patients exhibited decreased a-wave amplitude (a measure of photoreceptor function) while symptomatic, but not when symptoms were controlled. Studies have found a reduction in contrast sensitivity in schizophrenia (Keri et al., 2002) and this reduction was related to backward masking dysfunction (Slaghuis, 2004). On the other hand, other studies of visual processing in schizophrenia have demonstrated intact processing at the earliest visual stages. For example, patients and controls were comparable in the amount of contrast needed for target detection in a psychophysical procedure that was part of a masking task (Rassovsky et al., 2005). In addition, two recent studies have reported normal visible persistence in schizophrenia (Green et al., 2011b; Hahn et al., 2011).
The goal of the present study is to evaluate whether patients with schizophrenia exhibit impairments at the earliest, pre-perceptual stage of information processing. One way to assess very early visual processing (i.e., before the initial formation of the percept) is through a color priming paradigm. In one version of this task, a disk serves as a prime, and a ring that surrounds the disk appears slightly later. Subjects are asked to identify the color of the later-occurring ring. The disk and ring can be the same or different color. When the colors of the disk and ring are incongruent, it takes longer to identify the color of the ring than if the colors are congruent; referred to as a color priming effect. When the interval between the disk and the ring is short, the ring effectively masks the disk so that it is not consciously perceived. Notably, color priming occurs even if the disk is not consciously registered and this form of nonconscious color priming is thought to be due to pre-perceptual, stimulus-dependent levels of visual processing (Breitmeyer et al., 2004a; 2004b). Specifically, it involves the early feed forward sweep of information processing starting at the retina through V1 (Lamme et al., 2000) to higher levels in the cortical object recognition pathway (VanRullen, 2007; VanRullen & Thorpe, 2002). When the disk is visible (unmasked), it produces priming at multiple stages, including later, percept-dependent levels of cortical processing (Breitmeyer et al., 2007).
In the current study, we used color priming (Breitmeyer et al., 2004b) in a large sample of schizophrenia patients and healthy controls. We examined both conscious and nonconscious priming, and the focus was on nonconscious color priming because it provides a way to isolate the feed forward sweep. However, to our knowledge, this paradigm has not been used previously in schizophrenia research. If patients show a similar nonconscious priming effect (longer reaction times in the incongruent vs. congruent condition) to healthy controls, it would support the idea that they have intact visual processing at the very early feed forward stage before the formation of percepts.
Method
Participants
An initial sample of 181 patients with schizophrenia and 85 healthy comparison subjects participated in the study. Due to the exclusion criteria described below, data from 148 patients and 54 controls were used in the analyses. All participants were between the ages of 18 and 60, had an IQ over 70, were sufficiently fluent in English, were not actively using substances in the past 6 months, had no neurological disorder, seizures, history of head injury or loss of consciousness for more than one hour, and had normal color vision.
Patients with schizophrenia were recruited from outpatient treatment clinics at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) and from board-and-care residences in the community. Diagnosis was based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 1997). 76.2% of patients were receiving second-generation antipsychotic medications, 6.1% were on first-generation antipsychotic medications, 11.6% were on mixed antipsychotic medications, and 6.1% were not taking any antipsychotic medications at the time of testing.
Healthy controls were recruited through newspaper and internet advertisements and were screened with the SCID-I and SCID-II (First et al., 1996). They were excluded if they met criteria for any lifetime psychotic disorder, bipolar disorder, recurrent depression, substance dependence, paranoid, schizotypal, or schizoid personality disorder, or if they reported a history of psychotic disorder among their first-degree relatives.
All participants had the capacity to give informed consent and provided written informed consent after all procedures were explained in accordance with procedures approved by the Institutional Review Boards at the University of California, Los Angeles and VAGLAHS.
Clinical Ratings
Psychiatric symptoms during the month prior to testing were rated using the 24-item version of the Brief Psychiatric Rating Scale (BPRS; Lukoff et al., 1986) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984) by a trained rater. We report the “positive symptoms” and “depression/anxiety” factors, as well as the total score for the BPRS (Kopelowicz et al., 2008); for the SANS we report the global scores for affective flattening, alogia, anhedonia, and avolition (Table 1). All clinical assessments were conducted by interviewers trained to reliability through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) based on previously reported procedures (Ventura et al., 1993; 1998).
Table 1.
Demographic and Clinical Characteristics
| Healthy Controls (N=54) | Schizophrenia Patients (N=148) | |
|---|---|---|
| Age (Mean/SD)* | 41.31 (9.84) | 47.13 (9.06) |
| Gender (% Male) | 72.22% | 67.35% |
| Personal Education (Mean/SD)* | 14.32 (1.74) | 12.78 (1.92) |
| Parental Education (Mean/SD) | 13.23 (2.39) | 12.94 (2.93) |
| BPRS Total for 24 items (Mean/SD) | ---------- | 44.86 (10.13) |
| BPRS Positive Symptom (Mean/SD) | ---------- | 15.79 (6.57) |
| BPRS Depression/Anxiety (Mean/SD) | ---------- | 8.24 (2.94) |
| SANS Affective Flattening (Mean/SD) | ---------- | 1.49 (1.38) |
| SANS Alogia (Mean/SD) | ---------- | 0.69 (1.11) |
| SANS Avolition (Mean/SD) | ---------- | 2.89 (1.10) |
| SANS Anhedonia (Mean/SD) | ---------- | 2.60 (1.26) |
BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms.
Significant difference in age and personal education between healthy controls and patients, p < 0.001.
Color Priming Task
The experiment was conducted on a cathode ray tube monitor with a refresh rate of 160 Hz (6.25 ms per screen sweep). Participants were seated 100 cm from the computer monitor in a dimly lit room. All stimuli were presented using E-Prime 1.1 (Psychological Software Tools, Pittsburgh, PA).
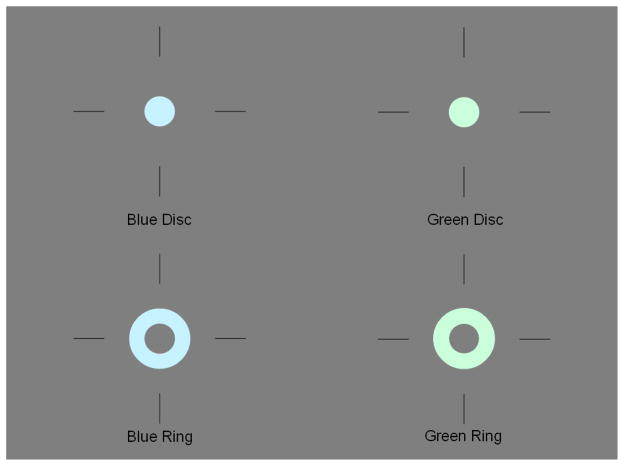
The procedure involved three different tasks, presented in separate blocks, in which the participant either identified the color of a disk or the color of a ring that surrounded the disk. The color of the disk and ring could be either green or blue. Participants were asked to quickly and accurately make their choice by using either the left or right button of a computer mouse for green or blue stimuli, respectively. The disk had a diameter of 8 mm (0.46 degrees of visual angle); the surrounding ring had an inner and outer diameter of 8 and 18 mm, respectively (0.46 and 1.03 degrees of visual angle). The disks and rings were either a desaturated green (red-blue-green [RBG] values = 202, 218, 254) or blue (RBG = 198, 254, 241) and were presented on a gray background (RBG = 127, 127, 127) within a fixation mark that consisted of four bars (2 cm for each bar) arranged in a notional cross (Breitmeyer et al., 2004b). The luminance of the gray background, green and blue stimuli was 12.6, 13.4, and 14.3 cd/m2, respectively. The disks were presented for 12.5 ms and the rings for 25.0 ms, with different stimulus onset asynchronies (SOAs) depending on the task. Examples of the stimuli were shown until participants were comfortable discriminating between the two colors. Figure 1 shows examples of the stimuli.
Figure 1.
Example of the stimuli used in the color priming paradigm. A green or blue ring follows a green or blue disk at a short (62.5 ms) or long SOA (200 ms).
The first task was a validity task in which participants were asked to identify the color of the disk that preceded the ring. This task was given to determine if the ring effectively masked the disk for each participant. Twelve practice trials were given with a relatively long SOA of 150 ms to familiarize participants with the task and to ensure they could differentiate between the colors of the disk at the duration they were shown. At this SOA, the ring did not mask the disk. After practice, 56 trials were given in which the ring followed the disk at an SOA of 62.5 ms, and participants were again asked to identify the color of the disk. The inter-trial interval varied with the participant’s reaction time, i.e., presentation of the next trial began 500 ms after the participant made a response. This validity task was used to exclude subjects who were not effectively masked at the 62.5 ms SOA. Specifically, we excluded participants who correctly identified the color of the disk in at least 37 of the 56 trials (p < 0.01 based on the binomial distribution). The SOA was selected based on pilot data that showed most subjects were masked at this interval.
Two other tasks, nonconscious and conscious priming, were administered in counterbalanced order across participants. For both tasks, the disk was presented before the ring, and participants identified the color of the ring and ignored the color of the disk. For nonconscious color priming, the SOA between the disk and the ring was 62.5 ms, which is suitable for masking. For conscious color priming, the SOA was 200 ms, which is too long for masking to occur. The color of the disk and ring were either congruent (both blue or both green) or incongruent (one blue and one green). There were 80 trials in each task (20 for each combination of disk-ring color: blue-blue, green-green, blue-green, green-blue). Only reaction times to correct responses to the color of the ring were analyzed.
For the nonconscious and conscious priming tasks, an incongruency score was calculated by subtracting the reaction time (RT) to the congruent from the RT to the incongruent condition. A score above 0 indicated a longer RT when the colors of the disk and ring were incongruent vs. congruent. As an additional check to make sure subjects could perform the task, we excluded from the analyses participants who had poor accuracy in the conscious color priming condition (i.e. scores < 48 out of 80 correct; p < 0.01 based on the binomial distribution). We considered performance at that level to be invalid.
Data analysis
For demographic variables, independent samples t-tests and chi-square tests were used to assess group differences for continuous and categorical variables, respectively. A 2 x 2 repeated measures analysis of variance (rmANOVA) with congruency (congruent vs. incongruent) as the within-subject factor and group as the between-subject factor was conducted to examine raw reaction time scores in the nonconscious condition. The same analysis was repeated for the conscious condition. We also conducted a 2 x 2 rmANOVA with condition (conscious vs. nonconscious) as the within-subject factor and group as the between-subject factor to investigate group differences in priming (incongruency scores). Significant interactions were decomposed using paired samples t-tests within each group. Finally, we performed Pearson’s correlations between the incongruency scores and symptom ratings within the patient group.
Results
Exclusion for Invalid Performance
First, in the validity condition, we excluded 19 out of 181 (10.5%) patients and 30 out of 85 (35.3%) controls who scored above the cut off, reflecting a lack of masking. Second, in the conscious priming condition, we excluded an additional 13 patients (7.2%) and no controls for poor performance as defined above. Lastly, we excluded one control and one patient who were outliers in terms of their incongruency scores (> 3 SDs above the mean and > 2 SDs relative to the next highest score). Therefore, the final sample sizes used in the analyses included 148 patients and 54 controls.
Demographic and Clinical Characteristics
Demographic and symptom ratings for the subjects in the final sample are shown in Table 1. There was a significant group difference in age (t [199] = 3.94, p < 0.001), with healthy controls being significantly younger than patients. Due to this age difference, we examined the correlation between age and each dependent variable. Age was not significantly associated with any of the variables of interest in either group, and was not considered in the analyses below. Groups did not differ in gender or parental education. The groups differed on personal education (t [198] = −5.14, p < 0.001), with patients having less education than healthy controls. We attempted to match the groups on parental, not personal, education. Patients exhibited mild to moderate levels of symptomatology.
Accuracy Results
After excluding subjects who did not exhibit masking, the mean number of trials in which the disk color was identified (out of a possible 56 trials) was 30.93 (2.96) for the healthy control group and 29.91 (3.18) for the patient group, with a range of 24 to 36 for both groups. Although this difference was significant at this sample size (t [200] = −2.05, p = 0.04), healthy controls correctly identified one more trial, on average, than patients.
We also examined the number of correct responses to the color of the ring (out of a possible 80 trials per task) for each of the color priming tasks. Independent samples t-tests revealed no significant differences between patients and healthy controls in the nonconscious condition (t [200] = −1.67, p = 0.10), 76.5 (4.2) and 77.6 (2.7), respectively. However, healthy controls had a significantly larger number of correct trials relative to patients in the conscious condition (t [200] = −3.05, p = 0.003), 78.2 (1.7) and 75.7 (6.1), respectively.
Nonconscious Color Priming
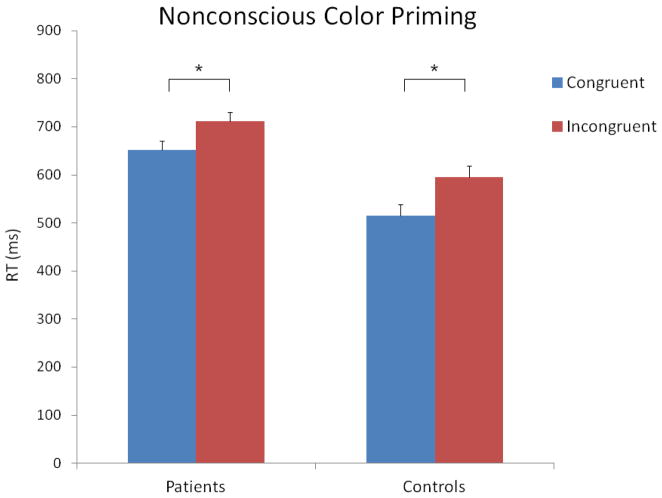
The 2 X 2 ANOVA with the raw reaction time data revealed significant main effects of congruency (F [1, 200] = 116.98, p < 0.001) and group (F [1, 200] = 13.19, p < 0.001). The main effect of congruency was due to longer RTs in the incongruent vs. congruent condition, 653 (246) and 583 (256) ms, respectively. The main effect of group was due to longer RTs in the patients vs. controls, 681 (218) and 555 (218) ms, respectively. The congruency X group interaction was not significant (F [1, 200] = 2.53, p = 0.11). These results are shown in Figure 2.
Figure 2.
Mean reaction times for the congruent and incongruent trials in the nonconscious priming condition. The vertical bars indicate standard errors. The stars indicate significant differences.
Conscious Color Priming
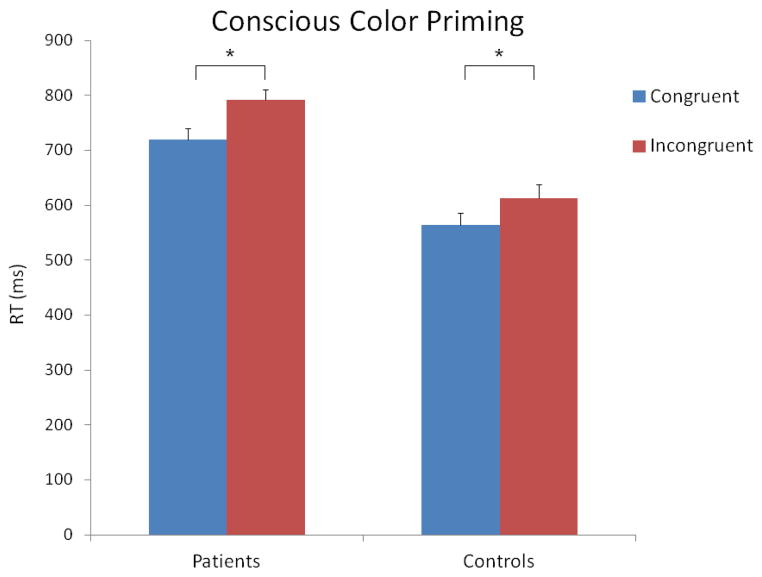
The 2 X 2 ANOVA with the raw reaction time data revealed significant main effects of congruency (F [1, 200] = 65.38, p < 0.001) and group (F [1, 200] = 22.60, p < 0.001). Similar to the results for the nonconscious condition, the main effect of congruency was due to longer RTs in the incongruent vs. congruent condition, 702 (260) and 642 (250) ms, respectively. The main effect of group was due to longer RTs in the patients vs. controls, 756 (221) and 589 (221) ms, respectively. The congruency X group interaction was not significant (F [1, 200] = 2.21, p = 0.14). These results are shown in Figure 3.
Figure 3.
Mean reaction times for the congruent and incongruent trials in the conscious priming condition. The vertical bars indicate standard errors. The stars indicate significant differences.
Incongruency Scores
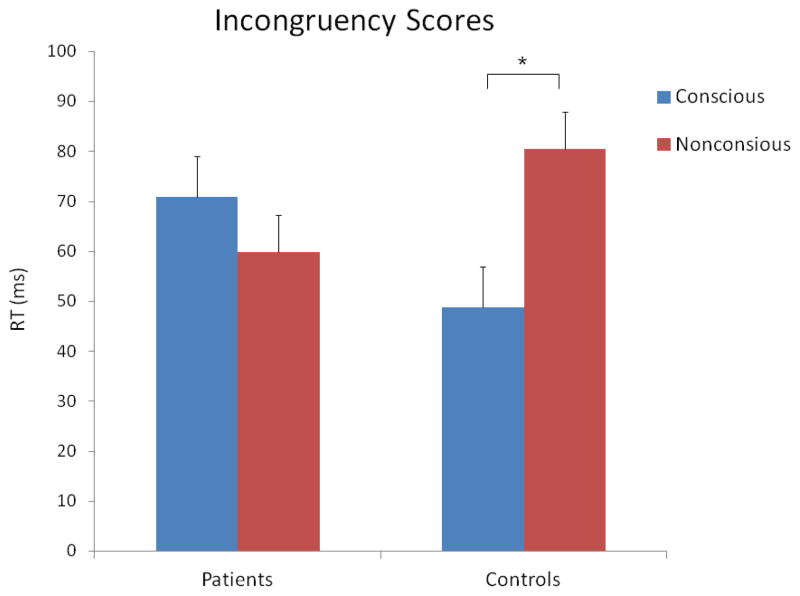
The 2 X 2 ANOVA with the incongruency scores revealed no significant main effects of consciousness (F [1, 200] = 1.07, p = 0.30) or group (F [1, 200] = 0.005, p = 0.94). However, the consciousness X group interaction was significant (F [1, 200] = 4.58, p = 0.03). Follow-up paired t-tests revealed that the interaction was due to healthy controls having a significantly larger priming effect in the nonconscious vs. conscious condition, 81 (46) and 49 (71) ms, respectively (t [53] = 2.72, p = 0.01). Patients showed the opposite pattern: a non-significantly larger priming effect in the conscious versus nonconscious condition, 71 (100) and 60 (91) ms, respectively (t [147] = −0.98, p = 0.33). These results are shown in Figure 4.
Figure 4.
Mean incongruency scores for the conscious and nonconscious conditions, in each group. The vertical bars indicate standard errors. The star indicates a significant difference.
Correlations between Symptoms and Behavioral Performance
We did not find any significant correlations at the p < 0.01 level between the incongruency scores (for the conscious and nonconscious conditions) and the symptom ratings, specifically BPRS Total, BPRS positive symptoms, BPRS depression/anxiety, SANS affective flattening, SANS alogia, SANS anhedonia, and SANS avolition.
Discussion
In this paper, we examined early, pre-perceptual stages of visual information processing using a behavioral color priming paradigm in a large sample of patients with schizophrenia compared to healthy controls. We focused on assessing the very early feed forward visual processing stage before the formation of percepts. In nonconscious color priming, we found evidence of priming (i.e. longer reaction times in the incongruent vs. congruent trials) to roughly the same extent in both groups. Given this finding, our results suggest that the stimulus-dependent, feed forward sweep of information processing might be intact in schizophrenia. That is, feed forward connections that convey information from the retina to lateral geniculate nucleus (LGN) to primary visual cortex (V1) do not seem to be disrupted in schizophrenia in this task. These results imply that the well-documented visual processing deficits in schizophrenia are likely due to abnormalities beyond the feed forward sweep. They may occur at later, percept-dependent, cortico-cortical stages of processing.
Regarding the conscious condition, results were similar to the nonconscious condition: Patients showed a conscious color priming effect similar to healthy controls. The neural processes underlying the intact priming effect in this condition is harder to interpret because conscious priming could involve multiple mechanisms, including feed forward, feedback or re-entrant activity from higher visual cortical areas (e.g., V4), as well as strategic influences.
Our findings also revealed that healthy controls had a significantly larger priming effect in the nonconscious vs. conscious condition; a reversed pattern (non-significantly larger priming in conscious vs. nonconscious condition) was observed in patients. Given that several prior studies in non-clinical samples (Tapia et al., 2010) showed stronger priming effects when primes were consciously than nonconsciously processed, the pattern in our controls was not expected. However, the finding is consistent with a previous report of non-clinical subjects showing nonsignificant priming effects with consciously vs. nonconsciously processed stimuli (Ro & Singhal, 2000). The absence or weakness of conscious priming effects may have been due to conscious control processes that conflict with and override automatic response tendencies (Morsella, 2005). It is possible that, in subjects who were visually aware of the prime, a conscious response strategy was adopted that produced somewhat faster responses in the incongruent conditions (Ro et al., 2009). Hence, the finding in patients of a greater priming in the conscious as compared to nonconscious condition, reflecting the more commonly-reported finding (Tapia & Breitmeyer, 2011; Tapia et al., 2010; 2011), might suggest that schizophrenia patients did not adopt conscious response strategies used by controls in this study to override automatic response tendencies.
Our results have implications in terms of visual pathways. Visual information is conveyed in two cortical processing streams; the ventral and dorsal cortical pathways that are dominated by parvocellular (P) and magnocellular (M) input, respectively. These pathways have differential properties; for example, M cells are not highly responsive to chromatic (color) contrast, while P cells are (Merigan & Maunsell, 1993). Because the primes and probes in the current study were luminance changes on an intermediate neutral background, they likely would have activated both M and P channels. However, because the color priming task relied specifically on the processing of chromatic information, the processing in the color-selective P channels was critically important. The color-blind M pathway is not relevant to the current task. Thus, our finding of normal nonconscious color priming in schizophrenia patients indicates intact P pathway function, consistent with a number of studies demonstrating M pathway dysfunction with relatively intact P processing in schizophrenia (Cadenhead et al., 1998; Green et al., 1994; Kim et al., 2006; Martinez et al., 2008; Schechter et al., 2003), although see others for exceptions (Keri et al., 2002; Slaghuis, 1998). In addition, two electrophysiological studies with schizophrenia patients specifically manipulated chromatic stimuli to differentially activate the M and P pathways (Butler et al., 2001; Schechter et al., 2005). In both studies, schizophrenia patients showed impairments in conditions that biased processing toward the M pathway (i.e., achromatic stimuli) but not in conditions emphasizing the P pathway (i.e., chromatic stimuli).
Finally, our findings can be viewed within a prominent theory on the pathophysiology of schizophrenia: abnormalities in the gamma amino butyric acid (GABA) system. GABA is involved in the modulation of visual processing and GABA interneuron abnormalities are present in the visual cortex in schizophrenia (Hashimoto et al., 2008). It has been proposed that these abnormalities might become more prominent as one moves up the processing hierarchy from V1 to the lateral occipital complex (Green et al., 2011a). That is, these abnormalities might not be very noticeable in the early feed forward processing stages (at the level of the LGN or V1) because GABA modulated lateral inhibition is not needed for the simple registration of brightness, color, line orientation, motion, and depth cues. However, the importance of GABA increases with higher-level visual processes where GABA inhibition is necessary for the processing of more complex visual stimuli, as well as contour integration. Hence, the GABA theory of schizophrenia could explain why a very early visual processing stage (i.e., the feed forward sweep) might be intact, even though later stages involved with object detection might be dysfunctional.
The study has several limitations. First, the majority of our patient sample was receiving antipsychotic medications at the time of testing which raises the question of medication effects. However, potential effects of medication on the present results are unlikely given the lack of group differences in priming effects. Moreover, previous studies of visual masking in schizophrenia found no effects of antipsychotic medications (Butler et al., 1996; Green et al., 1999). Second, our sample consisted of chronic patients and therefore would not be representative of recent-onset schizophrenia. Third, we did not formally test for color vision, which could have potentially affected our results. Nevertheless, both groups showed over 94% accuracy in identifying the color of the ring during the color priming tasks, suggesting that color vision dysfunction did not influence the results. Despite these limitations, results from this study are consistent with a growing literature on well-defined perceptual and cognitive processes that are intact in schizophrenia (Gold et al., 2009; Horan et al., 2012; Lee et al., in press).
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIH Grants MH043292 and MH065707 (MFG). The NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The authors would like to thank Cory Tripp, Poorang Nori, Mark McGee, Christen Waldon, and Amanda Bender for their assistance with recruitment and testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Balogh Z, Benedek G, Keri S. Retinal dysfunctions in schizophrenia. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008;32:297–300. doi: 10.1016/j.pnpbp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H, Chen J. Unconscious priming by color and form: different processes and levels. Consciousness and Cognition. 2004a;13:138–157. doi: 10.1016/j.concog.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ro T, Ogmen H, Todd S. Unconscious, stimulus-dependent priming and conscious, percept-dependent priming with chromatic stimuli. Perception and Psychophysics. 2007;69:550–557. doi: 10.3758/bf03193912. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ro T, Singhal NS. Unconscious color priming occurs at stimulus- not percept-dependent levels of processing. Psychological Science. 2004b;15:198–202. doi: 10.1111/j.0956-7976.2004.01503009.x. [DOI] [PubMed] [Google Scholar]
- Brittain P, Ffytche DH, McKendrick A, Surguladze S. Visual processing, social cognition and functional outcome in schizophrenia. Psychiatry Research. 2010;178:270–275. doi: 10.1016/j.psychres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Butler PB, DeSanti LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophrenia Research. 2002;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Butler PB, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biological Psychiatry. 1996;40:295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the VBM deficits of schizophrenia patients. Biological Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Archives of General Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Biometrics Research Department. New York, NY: New York State Psychiatric Institute; 1996. Structured Clinical Interview for DSM-IV Avis II Personality Disorders. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York, NY: New York State Psychiatric Institute; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. [Google Scholar]
- Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychology review. 2009;19:294–311. doi: 10.1007/s11065-009-9098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Lee J, Wynn JK, Mathis KI. Visual masking in schizophrenia: overview and theoretical implications. Schizophrenia Bulletin. 2011a;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. American Journal of Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: Specifying the visual channels. Archives of General Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Green MF, Wynn JK, Breitmeyer B, Mathis KI, Nuechterlein KH. Visual masking by object substitution in schizophrenia. Psychological Medicine. 2011b;41:1489–1496. doi: 10.1017/S003329171000214X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Kappenman ES, Robinson BM, Fuller RL, Luck SJ, Gold JM. Iconic decay in schizophrenia. Schizophrenia Bulletin. 2011;37:950–957. doi: 10.1093/schbul/sbp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. American Journal of Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, Green MF. Intact motivated attention in schizophrenia: evidence from event-related potentials. Schizophrenia Research. 2012;135:95–99. doi: 10.1016/j.schres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neurosciences. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Lee J, Harvey P-O, Horan WP, Kee K, Ochsner K, Penn D, Green MF. An intact social cognitive process in schizophrenia: situational context effects on perception of facial affect. Schizophrenia Bulletin. doi: 10.1093/schbul/sbs063. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Appendix A: Manual for the Expanded Brief Psychiatric Rating Scale (BPRS) Schizophrenia Bulletin. 1986;12:594–602. [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. The Journal of Neuroscience. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annual Review of Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Morsella E. The function of phenomenal states: supramodular interaction theory. Psychological Review. 2005;112:1000–1021. doi: 10.1037/0033-295X.112.4.1000. [DOI] [PubMed] [Google Scholar]
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biological Psychiatry. 2009;65:1094–1098. doi: 10.1016/j.biopsych.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer BG, Mintz J. Visual processing in schizophrenia: Structural equation modeling of visual masking performance. Schizophrenia Research. 2005;78:251–260. doi: 10.1016/j.schres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychological Medicine. 2011;41:487–497. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro T, Singhal NS, Breitmeyer BG, Garcia JO. Unconscious processing of color and form in metacontrast masking. Attention, Perception and Psychophysics. 2009;71:95–103. doi: 10.3758/APP.71.1.95. [DOI] [PubMed] [Google Scholar]
- Rund BR, Egeland J, Sundet K, Asbjornsen A, Hugdahl K, Landro NI, Lund A, Roness A, Stordal KI. Early visual information processing in schizophrenia compared to recurrent depression. Schizophrenia Research. 2004;68:111–118. doi: 10.1016/S0920-9964(03)00193-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clinical Neurophysiology. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophrenia Research. 2002;59:233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. Journal of Abnormal Psychology. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Experimental Brain Research. 2004;156:196–211. doi: 10.1007/s00221-003-1771-3. [DOI] [PubMed] [Google Scholar]
- Tapia E, Breitmeyer BG. Visual consciousness revisited: magnocellular and parvocellular contributions to conscious and nonconscious vision. Psychological Science. 2011;22:934–942. doi: 10.1177/0956797611413471. [DOI] [PubMed] [Google Scholar]
- Tapia E, Breitmeyer BG, Broyles EC. Properties of spatial attention in conscious and nonconscious visual information processing. Consciousness and Cognition. 2011;20:426–431. doi: 10.1016/j.concog.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Tapia E, Breitmeyer BG, Shooner CR. Role of task-directed attention in nonconscious and conscious response priming by form and color. Journal of Experimental Psychology Human Perception and Performance. 2010;36:74–87. doi: 10.1037/a0017166. [DOI] [PubMed] [Google Scholar]
- VanRullen R. The power of the feed-forward sweep. Advances in Cognitive Psychology. 2007;3:167–176. doi: 10.2478/v10053-008-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Thorpe SJ. Surfing a spike wave down the ventral stream. Vision Research. 2002;42:2593–2615. doi: 10.1016/s0042-6989(02)00298-5. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: 'The Drift Busters'. International Journal of Methods in Psychiatric Research. 1993;3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]