Abstract
Objective
To evaluate neurotrophin (NT) expression in the endometrium of women with and without endometriosis
Design
Prospective, cross-sectional, translational study
Setting
Academic hospital
Patient(s)
Thirty-three reproductive age women undergoing laparoscopy for infertility, pelvic pain, intramural fibroids, or tubal ligation
Intervention(s)
Endometrial biopsies, protein microarrays, RT-PCR, ELISAs and Western blotting
Main Outcome Measures
Neurotrophin proteins and mRNAs in eutopic endometrial biopsies
Results
Among seven neurotrophic proteins detected on the antibody microarrays, RT-PCR analysis confirmed nerve growth factor (NGF), NT-4/5, and brain-derived neurotrophic factor (BDNF) mRNAs in endometrial tissue. Quantitative ELISAs revealed that NT-4/5 (806 ± 701 vs. 256 ± 190 pg/100 mg protein, P=0.04) and BDNF (121 ± 97 vs. 14 ± 11 ng/100 mg protein, P<0.01) concentrations were significantly higher in women with endometriosis. NGF (100 ± 74 vs. 93 ± 83 pg/100 mg protein) levels did not differ between cases and controls (P=0.83).
Conclusions
Neurotrophins are synthesized in situ within the endometrium. NT-4/5 and BDNF proteins were more concentrated in biopsies from endometriosis cases than controls, whereas NGF levels were similar. We hypothesize that the local production of NTs induces sensory innervation of endometrium of women with endometriosis. These NTs represent novel targets for the diagnosis and treatment of endometriosis.
Keywords: nerves, endometriosis, proteomics, endometrium, pro-neurotrophins
INTRODUCTION
Endometriosis is defined as the presence of endometrial glands and stroma outside the uterine cavity. This benign gynecologic disorder affects ten to fifteen percent of reproductive age women (1, 2) and causes infertility, dysmenorrhea, dyspareunia, dyschezia, and chronic pelvic pain. The prevalence and the significant morbidity of the disease makes endometriosis a serious global public health problem (3), yet there remains a particular lack of understanding regarding the mechanisms of endometriosis-associated pain (4).
Microscopic studies have documented nerve fibers in peritoneal endometriotic implants (4–7), deep infiltrating endometriosis (8, 9), endometriosis related adhesions (10), and ovarian endometriomas (11). Recently, two independent investigative groups have demonstrated dense networks of nerve fibers in the superficial eutopic (intrauterine) endometrium of patients with endometriosis, whereas only rare nerves were noted in women without the disease (12–14). Zhang et al. (15) questioned the specificity of nerve fiber growth in endometriosis when they also identified neuronal markers in endometrium from patients with adenomyosis and fibroids. Nevertheless, these nerves remain highly (>90%) sensitive (12, 14, 16) for identifying endometriosis. The apparent differences in endometrial nerve fiber density between normal women and those with endometriosis provide a new mechanism to explain the pain associated with endometriosis and might afford new opportunities to diagnose endometriosis by their presence (14, 16, 17).
The mechanism(s) of endometrial neurogenesis in endometriosis patients remain(s) unknown. Research involving nerve growth in other areas of mammalian biology has identified several neurotrophins (NTs), a family of proteins that promote growth and survival of neurons (18). Nerve growth factor (NGF) was the first NT identified and is the most widely studied (19, 20). NGF has been localized in different types of endometriosis lesions (5, 9). Recently NGF, NT-3, NT-4/5 and brain-derived neurotrophic factor (BDNF) mRNA expression was identified in ovarian endometriomas and eutopic endometrium of women with Stage IV endometriosis and pain (21). However, quantitative protein evaluation in the eutopic endometrium has not been studied and comparisons of protein levels in endometriosis and control populations have not been performed.
We have hypothesized that neuro- and angio-trophic mitogens collude to stimulate coordinated nerve and blood vessel invasion in endometriosis. The co-expression of these factors, which we have termed “neuroangiogenesis,” could predispose the ectopic implantation and innervation of refluxed endometrial fragments (22). The present study used a proteomic approach to evaluate NT expression in eutopic endometrium and was validated with confirmatory methods.
We identified several NTs in the eutopic endometrium and quantified the protein concentrations of three NTs that are endogenously expressed; controls and subjects with endometriosis were compared. We demonstrate significant elevations in NT-4/5 and BDNF levels in subjects with endometriosis. The differences in NT concentrations in the eutopic endometrium indicate potential modulators of neuronal growth into the endometrium of endometriosis patients, which may contribute to pain symptoms in this patient population.
MATERIALS AND METHODS
Patient Selection
Thirty-seven reproductive age women (18–45 years) undergoing gynecologic surgery for infertility, pelvic pain, tubal ligation, or intramural fibroids were recruited at an academic hospital between July 2008 and February 2009. Subjects were enrolled from infertility and general gynecology practices to enhance diversity of the patient population. Women who had been on estrogen or progesterone containing medications or other forms of pituitary suppression in the previous three months, pregnant women or those that had not resumed normal menstruation following delivery, and women who were unable to understand or give written consent prior to participating, were excluded. This study was approved by the Emory and Wake Forest University Institutional Review Boards.
After providing written informed consent, each participant answered a questionnaire to evaluate their eligibility and identify the presence of infertility and pelvic pain characteristics. Patients were classified as infertile if they had been actively trying to achieve a pregnancy for at least 12 months. We also asked patients if they had experienced pelvic pain in the past three months and if so, whether it was severe enough to affect their activities of daily living (AoDL).
At laparoscopy, a comprehensive visual evaluation of the pelvis, cul-de-sac and diaphragmatic domes was undertaken by experienced gynecologic surgeons familiar with typical and atypical manifestations of endometriosis, including vascular and vesicular lesions. Following the surgery, women were classified as having endometriosis (n=21) if their surgeon noted laparoscopic evidence of endometriosis lesions. Women were classified as controls (n=16) if there was no visible evidence of endometriosis. If only peritoneal defects or filmy adhesions were observed, the subjects were considered controls. In cases where biopsies were submitted for pathologic review, histological confirmation was verified in ~80% of suspicious lesions, consistent with the literature (23), but the clinical classification was not changed based on pathological reports.
Tissue collection
Eutopic endometrial biopsy specimens were collected after induction of general anesthesia via a 1.5 mm silastic Pipelle® (CooperSurgical, Inc., Trumbull, CT) catheter, immediately prior to placement of an intrauterine manipulator. Efforts were made to obtain fundal tissue. Sampling at either proliferative or secretory cycle phases was allowed based on the findings of Al-Jefout et al. (16) documenting the presence of functionalis zone nerves in women with endometriosis independent of the phase of the menstrual cycle. Tissue was blotted dry, divided into two equal portions, one in a tube containing RNAlater® (Qiagen, Valencia, CA), snap frozen in liquid nitrogen and stored at −80 C.
Biochemical evaluations
Fresh frozen biopsy aliquots were thawed on ice and subjected to one the following analytical procedures.
For protein microarray, ELISAs and Western blotting, freshly thawed biopsy fragments were placed in 300 μl of lysis buffer (50 mM Tris, pH 7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1% Nonidet P40, 0.02% NaN3) and homogenized by vortexing until the tissue was dispersed. The samples were then placed on ice for 30 minutes before centrifugation (14,000 rpm for 15 minutes). Total protein concentrations in the homogenate supernatants were quantified by the bicinchoninic acid protein assay kit (Sigma Chemical Co., St. Louis, MO) and the results used for normalization of microarrays, ELISAs and Western blots.
Pilot studies were performed using a subset of endometrial biopsies from endometriosis (n=4) and control (n=4) subjects to determine which NTs were present in the endometrium. An extended prototype protein microarray was performed at RayBiotech® Inc. (Norcross, GA). The assay was performed as described in their previous publications (24, 25). Briefly, array membranes with immobilized capture antibodies were blocked with 5% bovine serum albumin (BSA)/TBS (0.01 M Tris HCl pH7.6/0.15 M NaCl) for 1 hour. Membranes were then incubated with 1 ml of 10-fold diluted endometrial lysates prepared in 5% BSA/TBS for 2 hours at room temperature. After extensive washing with TBS/0.1% Tween 20 (3 times, 5 min each) and TBS (2 times, 5 min each) to remove unbound cytokines and growth factors, membranes were then incubated with a cocktail of biotin-conjugated anti-cytokine and growth factor antibodies. Washing followed and membranes were incubated with horseradish peroxidase-conjugated streptavidin (2.5 pg/ml) for 1 hour at room temperature. Unbound reagents were washed with TBS/0.1% Tween 20, followed by TBS. Finally, the signals were detected using the Enhanced Chemiluminescence (ECL) System (Amersham Pharmacia Biotech, Piscataway, NJ).
A total of seven neurotrophic proteins (NGF, NT-3, NT-4/5, BDNF, ciliary neurotrophic factor [CNTF], glial cell derived neurotrophic factor [GDNF] and neurotrophin receptor p75 [NTR]) were detected in the microarrays. Based on the microarray signal intensity and reports of NT expression in the literature (21, 26), we selected the three most prominent (NGF, NT-4/5 and BDNF), for RT-PCR analyses, quantitative ELISAs and Western blotting.
Reverse transcription-PCR (RT-PCR)
To validate the de novo biosynthesis of NTs within the endometrium and distinguish their generation from post-translational accumulation within the tissue, total RNA was extracted from frozen biopsy samples stored in RNAlater® and RT-PCR with sequence-specific primers was performed. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), using intron-spanning primers that rule out genomic DNA contamination (27), was used as an internal control of mRNA quantity and integrity.
After preparation of complementary DNA, NGF-specific primers (5′-TGAAGCTGCAGACACTCAGG-3′ [sense], 5′GACAAAGGTGTGAGTCGTGGT-3′ [antisense], amplicon=340 base pairs [bp]) were used for amplification. 2 μL cDNA and 0.6 μM specific primers were added to supermix reagents (Bio-Rad, Hercules, CA) and end-point PCR amplification was performed on a DNA Engine Opticon 2 system (Bio-Rad) according to the previously described method (28). GAPDH primers (5′ TGATGACATCAAGAAGGTGGTGAA-3′ [sense] and 5′TCCTTGGAGGCCATGTGGGCCAT-3′ [antisense], amplicon=185 bp) were amplified as an internal control for mRNA quality. Primers for NT-4/5 and BDNF were commercially obtained from Qiagen (QT00210924 and QT00235368, yielding amplicons of 96 bp and 120 bp, respectively). The precise sequence information is proprietary. We performed end-point, non-quantitative RT-PCR to verify the presence of NT mRNA, and chose to quantify protein expression between the two groups with ELISAs as indicated below.
ELISAs
Based on our screening with the protein microarrays and confirmation by RT-PCR, quantitative ELISAs were performed to measure NGF, NT-4/5 and BDNF protein levels. Protein lysates, prepared as described above, were thawed and resuspended. Neurotrophin protein levels were measured by two-site, sandwich ELISAs using affinity-purified rabbit anti-human NGF and Emax® ImmunoAssay System reagents from Promega (Madison, WI) and anti-human NT-4/5 and anti-human BDNF ELISA kits from RayBiotech®. ELISAs were performed per the manufacturers’ instructions after acidification to detect total NT proteins. The sensitivities of the NGF, NT-4/5 and BDNF ELISAs were determined by the manufacturers to be 15 pg/ml, 2 pg/ml and 0.3 ng/ml, respectively. Each of the three ELISAs was highly specific for the corresponding analyte and had less than 0.1% cross-reactivity with other NTs or related cytokines. Neurotrophin concentrations were consistently within the linear range of the standard curve. Empirically derived coefficients of variation were less than 5% for the NGF assay and less than 12% for the NT-4/5 and BDNF assays. Neurotrophin concentration determinations were performed in duplicate for each specimen and are reported as pg or ng/100 mg of tissue lysate protein.
Western Blots
Frozen lysates, prepared as described previously were thawed and resuspended. A total of 60 μg of protein from each specimen were separated on 4–12% SDS-polyacrylamide gradient gels and transferred to PVDF paper. The membranes were blocked at room temperature for 1 hour in TBS/0.05% Tween-20 (TBS-T) with 5% non-fat milk and probed with affinity-purified rabbit polyclonal anti-NGF IgG (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, #SC-548, 1:200 dilution), mouse monoclonal anti-NT-4/5 (Abcam, Cambridge, MA, #87394, 1:500 dilution) and mouse monoclonal anti-BDNF (Abcam, #10505, 1:500 dilution) with rotation at 4 C overnight. Mouse monoclonal anti-β-actin (Sigma, St. Louis, MO, #A4700) antibodies at a dilution of 1:500 were used as an internal housekeeping control. After washing with TBS-T, membranes were incubated with goat anti-rabbit or mouse IgG secondary antibodies (Pierce Biotechnology, Rockford, IL) at a dilution of 1:20,000 for 1 h at room temperature. After further washing with TBS-T, bands were visualized by chemiluminescence using Kodak Biomax film.
Statistical analyses
The study population characteristics were evaluated using SAS version 9.1. Differences in age, history of infertility, and characteristics of pelvic pain were evaluated between the endometriosis and control groups. Student’s t-test was used to detect differences in continuous, normally-distributed characteristics. Cochran-Mantel-Haenszel tests were used to evaluate differences in binomial outcome characteristics. Sample size calculations for NT protein levels obtained by ELISA indicated that 15 subjects in each group would provide 80% power (1-β) to detect an 80% difference, based on the largest assay variance (BDNF) with a two-tailed α=0.05. Shapiro-Wilk and Kolmogorov-Smirnov tests revealed that the ELISA data were mostly normally distributed, however, given the relatively small sample sizes and the fact that some of the data were not normally distributed, statistical analyses using conservative nonparametric (Mann-Whitney U) tests were applied to compare differences between control and endometriosis groups. Data are presented as mean ± SD.
RESULTS
Study population
Thirty-seven patients recruited to participate in the study provided written informed consent prior to surgery. After careful laparoscopic inspection, 21 patients were observed to have endometriosis lesions and 16 had no clinical evidence of endometriosis (controls). Due to incomplete questionnaire data, inability to obtain tissue and loss of one specimen during transportation, a total of 33 specimens were evaluated (endometriosis: n=18; controls: n=15). The subjects ranged in age from 21–45 years. The mean age (± SD) of endometriosis patients was 34± 7 and that of controls was 34 ± 6 years. In the endometriosis population, 56% of the cohort reported being infertile, 80% reported dysmenorrhea, and 60% reported dysmenorrhea severe enough to affect AoDL. In the control group, 43% reported infertility, 57% reported dysmenorrhea, and 29% reported pain severe enough to affect AoDL. The following were the major operative findings in the 15 controls without evidence of endometriosis: five had intramural or subserosal fibroids, three had pelvic adhesions, three had tubal obstruction, two had no evidence of pelvic pathology and two underwent tubal sterilization. Given the indications for laparoscopy and high-risk nature of our clinical practices, it was not surprising that the presence of symptoms did not differ significantly between the two groups in any category. A higher proportion of subjects with endometriosis reported dyspareunia, dyschezia or severe pain (P=0.08), but the trend did not reach statistical significance. The proportion of patients with infertility or a family history of endometriosis also was similar between the two groups.
Antibody Microarrays
The pilot proteomic studies (endometriosis subjects [n=4], controls [n=4]) allowed us to identify several NTs to study further at the mRNA and protein expression levels. Among the seven NT proteins identified by protein microarray, NT-3, CNTF, GDNF and NTR signals approached those of the negative controls, suggesting that those four proteins were present at low concentrations in the endometrium. Hence, we selected NGF, NT-4/5, and BDNF for further study.
RT-PCR
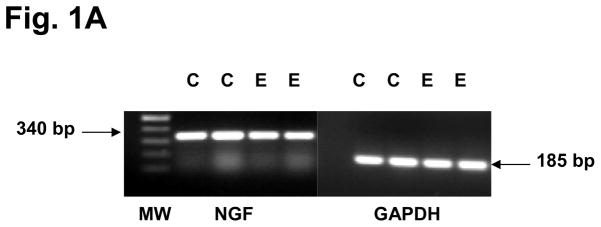
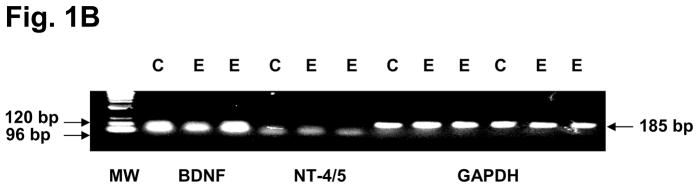
To confirm that the three eutopic endometrial NTs selected by antibody microarrays were a result of in situ biosynthesis, rather than production of the proteins outside the endometrium with subsequent accumulation within that tissue, RT-PCR was performed for NGF, NT-4/5, and BDNF mRNA. Amplicons of the expected sizes for all three NT transcripts were identified in the endometrial biopsies (Figs. 1A and B), confirming that their genes were endogenously expressed in situ. Fig. 1A represents findings from two independent controls and two independent endometriosis cases, but similar RT-PCR results were obtained in a minimum of five control and five endometriosis cases. Based on these RT-PCR findings, we proceeded to quantify NGF, NT-4/5 and BDNF levels in all the subjects using validated ELISAs for quantitative NT comparisons between the two groups.
Figure 1.
Figure 1A. RT-PCR confirmed the expression of NGF mRNA (predicted amplicon=340 bp) and GAPDH mRNA (predicted amplicon=185 bp) in eutopic endometrial biopsies from two independent and representative controls (C) and two independent endometriosis (E) subjects. Amplicon lengths are indicated as base pairs (bp) and correspond with the molecular weight (MW) ladder in the left lane.
Figure 1B. RT-PCR confirmed the expression of BDNF mRNA (predicted amplicon=120 bp), NT-4/5 mRNA (predicted amplicon=96 bp) and GAPDH mRNA (predicted amplicon=185 bp) in eutopic endometrial biopsies from a representative control (C) and two independent endometriosis (E) subjects. GAPDH amplification was performed in duplicate. Molecular weight (MW) ladder is shown at left.
ELISAs
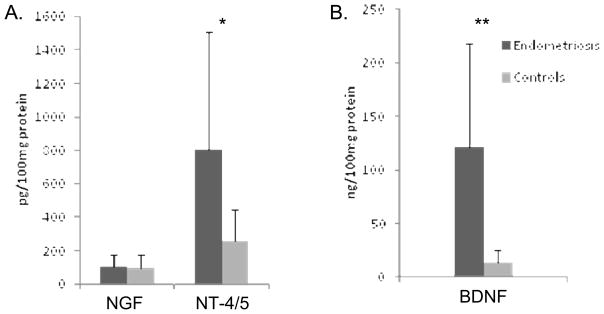
Total NT protein levels were measured using validated ELISAs for NGF, NT-4/5 and BDNF, and are expressed as pg or ng/100 mg protein to normalize the concentrations and allow accurate comparisons among the different samples. The NGF concentration in the endometriosis group was 100.0 ± 74.2 pg/100 mg protein, not significantly different from that in the controls 93.4 ± 83.0 pg/100 mg protein (P=0.83, Mann-Whitney U test). NT-4/5 and BDNF levels were significantly higher in the endometriosis cases than in the controls (806 ± 702 vs. 256 ± 190 pg/100 mg protein, P=0.04 and 121 ± 96.9 vs. 14 ± 11.2 ng/100 mg protein, P<0.01, respectively, Fig. 2).
Figure 2.
Figure 2A. Quantitative ELISAs of NGF and NT-4/5 proteins in lysates of endometrial biopsies from endometriosis (n=18) and control (n=15) subjects. Data are shown as mean ± SD (* P=0.04, Mann-Whitney U test).
Figure 2B. Quantitative ELISA of BDNF protein in lysates of endometrial biopsies from endometriosis (n=18) and control (n=15) subjects. Data are shown as mean ± SD (** P<0.01, Mann-Whitney U test).
Western blotting
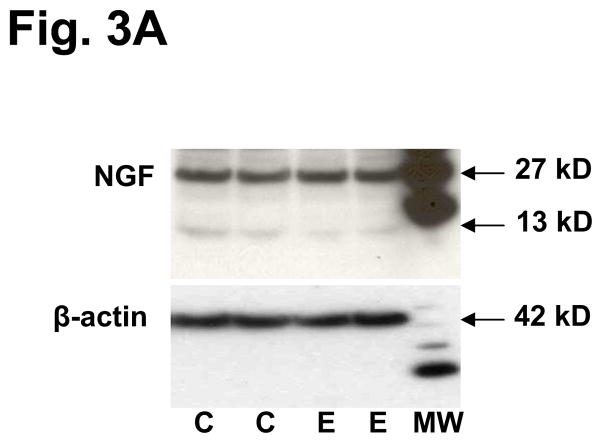
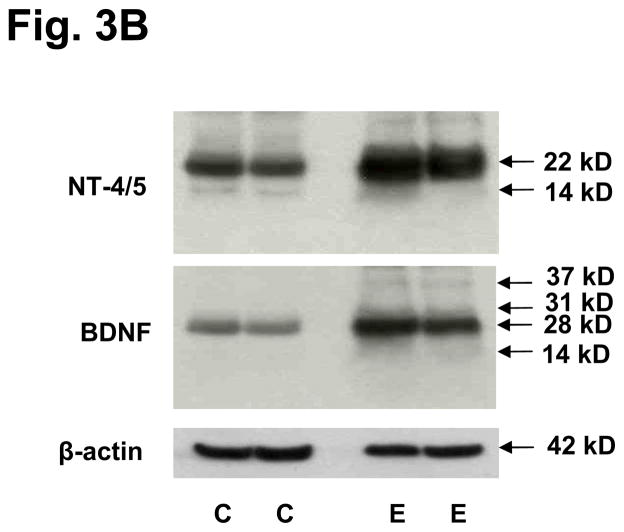
We performed Western blots on two independent and representative examples from each group to validate the molecular sizes of the NTs differentially expressed in eutopic endometrium. As observed in the ELISA data, concentrations of NGF did not differ between control and endometriosis cases (Fig. 3A). An interesting finding was that only a minority of the NGF migrated as the mature 13 kD isoform, whereas the majority had an apparent mass of 27 kD (Fig. 3A), corresponding to proNGF (29). As expected, levels of β-actin, a housekeeping control protein, were constant in controls and cases. Levels of NT-4/5 and BDNF were greater in endometriosis cases and their predominant species migrated as proNTs of 22–28 kD, with very little mature (14 kD) NTs detected in the endometrial lysates (Fig. 3B). Again, the levels of β-actin did not differ between controls and endometriosis cases.
Figure 3.
Figure 3A. Western blot of NGF in lysates of endometrial biopsies. Two representative controls (C) and endometriosis (E) cases are shown. Molecular weights are shown in kilodaltons (kD) as determined from the standard ladder shown at the right (MW). The faint band at 13 kD represents mature NGF processed from the 27 kD precursor. β-actin, a housekeeping control protein, was immunoblotted to verify equal loading.
Figure 3B. Western blots of NT-4/5 and BDNF in lysates of endometrial biopsies. Two representative controls (C) and endometriosis (E) cases are shown. Molecular weights are shown in kilodaltons (kD) at the right. The predominant bands of 22 kD and 28 kD represent proNT-4/5 and proBDNF, respectively. The faint bands at 14 kD represent mature, processed NT-4/5 and BDNF. β-actin, a housekeeping control protein, was immunoblotted to verify equal loading.
DISCUSSION
Innervation of endometriotic implants has been documented in all types of lesions in women and in experimental animal models of the disease (4–11). We have proposed that trophic stimuli induce coordinated nerve and blood vessel invasion of endometriotic implants; a process described in embryonic development and tissue remodeling that we refer to as “neuroangiogenesis” (22, 30). We postulated that increased NT production also would be present in the eutopic endometrium of endometriosis patients. Our hypothesis seemed plausible, as many examples of upregulated inflammatory and invasive genes are reported in this tissue (31, 32). Furthermore, messenger RNAs encoding the same NTs proteins that we describe in this paper were reported recently in endometriosis lesions (21). Previous reports of elevated levels of NGF levels in peritoneal fluid (33) and of the TrkB receptor in the eutopic endometrium of endometriosis cases (34), which preferentially binds NT-4/5 and BDNF, have been published.
Our current study was conceived based on the reports of Tokushige et al. (12) and Bokor et al. (14) that Aδ and C sensory nerve fibers could be detected in the superficial functionalis layer of the endometrium in almost all women with endometriosis, but not in women without the disorder. In recent publications from those groups, the specificity of endometrial nerve detection was reported to be 83% (16) and >90% (14) with a sensitivity >90% (14, 16). Endometrial nerve density does not change during the ovulatory cycle (16), suggesting that factors other than gonadotropins or ovarian steroid hormones are involved in their growth and regulation. Moreover, this finding indicates that detection of nerve fibers or neurotrophic factors need not be limited to a specific menstrual cycle phase, making clinical trials and ultimately minimally-invasive diagnostic practices much easier to conduct and operationalize. The observation also suggests that non-hormonal therapies might be capable of targeting endometrial neuroangiogenesis without the detrimental side-effects of disrupting the hypothalamic-pituitary-ovarian axis, a limitation of most current medical interventions for endometriosis (35).
Our clinical findings revealed a high prevalence of pelvic pain and infertility in both the endometriosis and control subjects, likely due to the high-risk, symptomatic population from which the participants were recruited. Unfortunately, the study was not powered to correlate NT protein concentrations with specific symptoms (e.g., dysmenorrhea), and stratification based on such findings was not revealing. The questionnaire was not a validated pain instrument and was used only to identify symptoms typically encountered by clinicians evaluating women with complaints suggestive of endometriosis. It is possible that more striking differences might have been observed if a histopathological diagnosis of endometriosis (rather than visual confirmation) was required for classification, or had a fertile control population without pain been used for comparison.
Although the presence of nerves within the endometrium of women with endometriosis appears to be highly sensitive and reproducible in multiple laboratories, if not entirely specific (15), we lack understanding as to why superficial nerves are restricted to subjects with disease. Our findings suggest that high local concentrations of NT-4/5 and BDNF, but not NGF, stimulate differential nerve fiber growth in cases relative to controls. Ultimately, the measurement of differentially-expressed NTs in endometrial biopsies could serve as adjuvant biomarkers, in combination with or as a substitute for immunohistological detection of endometrial neurons; the latter being promoted as a minimally-invasive endometriosis diagnostic test (14, 16, 17). Western blotting revealed that the predominant species of the NTs evaluated migrated as 22–28 kD precursor isoforms (36), with only trace amounts of the mature, processed peptides of 13–14 kD (Figs. 3A and B). We postulate that the biopsies reflect pools of intracellular NT precursors, rather than their processed, secreted products. Mature NTs are more likely to be secreted from cells, however with transfection and overexpression, pro-NTs also can be secreted into the extracellular milieu (37). This observation has important implications, as interference with NT processing within the endometrium may represent a completely novel therapeutic strategy for the reduction of mature NT production and potential symptom control. Future studies should explore whether NT biosynthesis or action can be targeted to mitigate the pain associated with endometriosis.
Acknowledgments
The authors thank Kara Barrett, RN for her tireless efforts in specimen collection and the subjects for their generous participation. This research was supported by the NIH Atlanta Clinical and Translational Science Institute UL1-RR25008 and UL1-RR25009 (to ASB and RNT), NIH STTR grant R41-HD65360 (to R-PH, NS and RNT) and a fellowship from the Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (to AMCF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winkel CA. Evaluation and management of women with endometriosis. Obstet Gynecol. 2003;102:397–408. doi: 10.1016/s0029-7844(03)00474-5. [DOI] [PubMed] [Google Scholar]
- 2.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 3.Bianconi L, Hummelshoj L, Coccia ME, Vigano P, Vittori G, Veit J, et al. Recognizing endometriosis as a social disease: the European Union-encouraged Italian Senate approach. Fertil Steril. 2007;88:1285–7. doi: 10.1016/j.fertnstert.2007.07.1324. [DOI] [PubMed] [Google Scholar]
- 4.Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006;21:3001–7. doi: 10.1093/humrep/del260. [DOI] [PubMed] [Google Scholar]
- 5.Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–900. doi: 10.1093/humrep/17.7.1895. [DOI] [PubMed] [Google Scholar]
- 6.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101:11094–8. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mechsner S, Kaiser A, Kopf A, Gericke C, Ebert A, Bartley J. A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers in peritoneal endometriotic lesions. Fertil Steril. 2009;92:1856–61. doi: 10.1016/j.fertnstert.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, et al. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod. 2000;15:1744–50. doi: 10.1093/humrep/15.8.1744. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod. 2009;24:827–34. doi: 10.1093/humrep/den464. [DOI] [PubMed] [Google Scholar]
- 10.Tulandi T, Chen MF, Al-Took S, Watkin K. A study of nerve fibers and histopathology of postsurgical, postinfectious, and endometriosis-related adhesions. Obstet Gynecol. 1998;92:766–8. doi: 10.1016/s0029-7844(98)00298-1. [DOI] [PubMed] [Google Scholar]
- 11.Tokushige N, Russell P, Black K, Barrera H, Dubinovsky S, Markham R, et al. Nerve fibers in ovarian endometriomas. Fertil Steril. 2010;94:1944–7. doi: 10.1016/j.fertnstert.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 12.Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006;21:782–7. doi: 10.1093/humrep/dei368. [DOI] [PubMed] [Google Scholar]
- 13.Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril. 2007;88:795–803. doi: 10.1016/j.fertnstert.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 14.Bokor A, Kyama CM, Vercruysse L, Fassbender A, Gevaert O, Vodolazkaia A, et al. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod. 2009;24:3025–32. doi: 10.1093/humrep/dep283. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril. 2009;92:1799–801. doi: 10.1016/j.fertnstert.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Al-Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, Markham R, et al. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod. 2009;24:3019–24. doi: 10.1093/humrep/dep275. [DOI] [PubMed] [Google Scholar]
- 17.Medina MG, Lebovic DI. Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand. 2009;88:968–75. doi: 10.1080/00016340903176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 19.Levi-Montalcini R, Cohen S. In vitro and in vivo effects of a nerve growth-stimulating agent isolated from snake venom. Proc Natl Acad Sci U S A. 1956;42:695–9. doi: 10.1073/pnas.42.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A. Update of the NGF saga. J Neurol Sci. 1995;130:119–27. doi: 10.1016/0022-510x(95)00007-o. [DOI] [PubMed] [Google Scholar]
- 21.Borghese B, Vaiman D, Mondon F, Mbaye M, Anaf V, Noel JC, et al. Neurotrophines et douleur: étude d’expression et de corrélation dans l’endométriose. Gynecol Obstet Fertil. 2010;38:442–6. doi: 10.1016/j.gyobfe.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–82. doi: 10.1146/annurev-physiol-012110-142158. [DOI] [PubMed] [Google Scholar]
- 23.Stegmann BJ, Sinaii N, Liu S, Segars J, Merino M, Nieman LK, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89:1632–6. doi: 10.1016/j.fertnstert.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang RP, Huang R, Fan Y, Lin Y. Simultaneous detection of multiple cytokines from conditioned media and patient’s sera by an antibody-based protein array system. Anal Biochem. 2001;294:55–62. doi: 10.1006/abio.2001.5156. [DOI] [PubMed] [Google Scholar]
- 25.Huang RP. Simultaneous detection of multiple proteins with an array-based enzyme-linked immunosorbent assay (ELISA) and enhanced chemiluminescence (ECL) Clin Chem Lab Med. 2001;39:209–14. doi: 10.1515/CCLM.2001.032. [DOI] [PubMed] [Google Scholar]
- 26.Giannini A, Bucci F, Luisi S, Cela V, Pluchino N, Merlini S, et al. Brain-derived neurotrophic factor in plasma of women with endometriosis. J Endometriosis. 2010;2:144–50. [Google Scholar]
- 27.Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol. 2011;344:25–34. doi: 10.1016/j.mce.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toti P, Ciarmela P, Florio P, Volpi N, Occhini R, Petraglia F. Human placenta and fetal membranes express nerve growth factor mRNA and protein. J Endocrinol Invest. 2006;29:337–41. doi: 10.1007/BF03344105. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zhang SF, Zou SE, Xia X, Bao L. Accumulation of nerve growth factor and its receptors in the uterus and dorsal root ganglia in a mouse model of adenomyosis. Reprod Biol Endocrinol. 2011;9:30. doi: 10.1186/1477-7827-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker BA, Mearow KM. Peripheral sensory axon growth: from receptor binding to cellular signaling. Can J Neurol Sci. 2008;35:551–66. doi: 10.1017/s0317167100009331. [DOI] [PubMed] [Google Scholar]
- 31.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–47. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- 33.Hou Z, Sun L, Gao L, Liao L, Mao Y, Liu J. Cytokine array analysis of peritoneal fluid between women with endometriosis of different stages and those without endometriosis. Biomarkers. 2009;14:604–18. doi: 10.3109/13547500903183970. [DOI] [PubMed] [Google Scholar]
- 34.Anger DL, Zhang B, Boutross-Tadross O, Foster WG. Tyrosine receptor kinase B (TrkB) protein expression in the human endometrium. Endocrine. 2007;31:167–73. doi: 10.1007/s12020-007-0025-8. [DOI] [PubMed] [Google Scholar]
- 35.Wieser F, Cohen M, Gaeddert A, Yu J, Burks-Wicks C, Berga SL, et al. Evolution of medical treatment for endometriosis: back to the roots? Hum Reprod Update. 2007;13:487–99. doi: 10.1093/humupd/dmm015. [DOI] [PubMed] [Google Scholar]
- 36.Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, et al. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–60. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farhadi HF, Mowla SJ, Petrecca K, Morris SJ, Seidah NG, Murphy RA. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J Neurosci. 2000;20:4059–68. doi: 10.1523/JNEUROSCI.20-11-04059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]