FIGURE 3. ROP2/RIC4 inhibition of PIN1 endocytosis is analogous to Cdc42/Par inhibition of endocytosis in Drosophila.
3 A
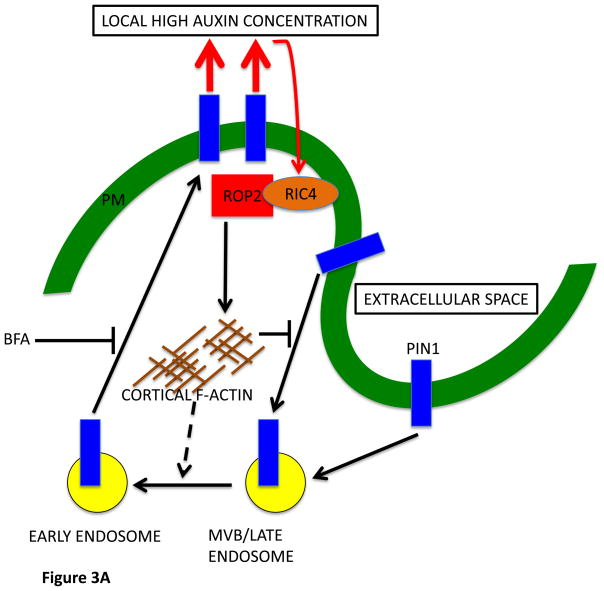
A model for PIN1 polarization to the lobe region of PCs via a ROP signaling-based feed-forward mechanism.
ROP2 in the lobing region is activated by extracellular auxin, and the activated ROP2-RIC4 pathway leads to inhibition of PIN1 internalization through RIC4-dependent cortical F-actin, causing PIN1 polarization to the lobing region. The PIN1-based export of auxin causes additional ROP2 activation to complete this feed-forward cycle. Recently published data implies a role for the activated ROP2-RIC4 pathway in the promotion of endosomal trafficking from Ara7-marked endosomes to recycling endosomes [41]. Ara7 is a Rab5 homolog and resides in an endosomal compartment from which many internalized proteins, including PIN1, are sorted for targeting to the vacuole or recycling to the PM.
3 B
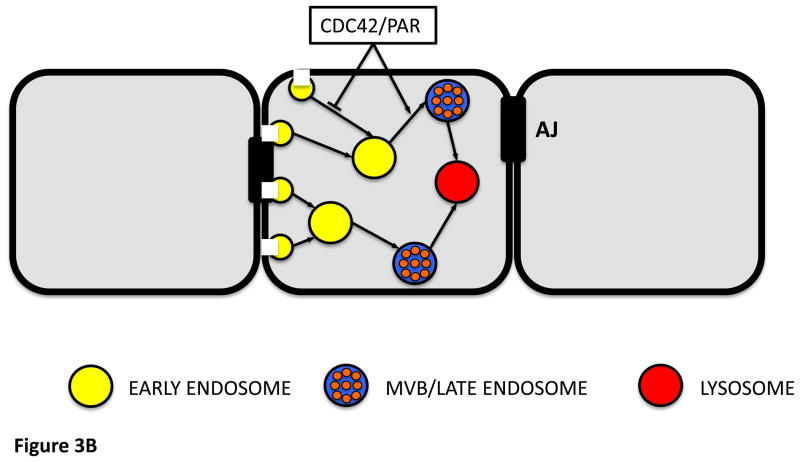
A model for the regulatory function of the Cdc42-Par complex in the apical endocytic pathway.
Recent data [97] indicate that Cdc42 and the Par complex cooperate in regulating two distinct steps in the endocytosis of apical membrane components. Endocytosis from the plasma membrane is inhibited by the Cdc42–Par complex, which in turn promotes the progression of early endosomes (EE) to the multivesicular bodies/late endosomes (MVBs/LEs). Apical and basolateral proteins, including adherens junction (AJ) proteins, follow distinct endocytotic routes.